Preparation method of rupatadine fumarate impurity S
A technology for rupatadine fumarate and impurities, which is applied in the field of pharmaceutical synthesis, can solve the problems such as the preparation method of rupatadine fumarate impurity S and the like are not proposed, and achieves low price, good reproducibility, and improved yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0042] The preparation method includes:
[0043] Substitution reaction: carry out substitution reaction between raw material A and raw material B to obtain a substituted product, raw material A has the structure shown in formula (I), raw material B has the structure shown in formula (II), and the substituted product has the structure shown in formula (III) ,
[0044]
[0045] x 2 is a halogen atom;
[0046] Halogenation reaction: the substitution product is subjected to a halogenation reaction with a halogenation reagent to obtain a halogenation product, which has a structure shown in formula (IV),
[0047] x 1 is a halogen atom; and
[0048] Condensation reaction: react the halogenated product with raw material C to obtain rupatadine fumarate impurity S, raw material C has the structure shown in formula (Ⅴ),
[0049]
[0050] Specifically, the synthetic route is:
[0051]
[0052] Based on the inventor's many years of work experience, the present application ...
Embodiment 1
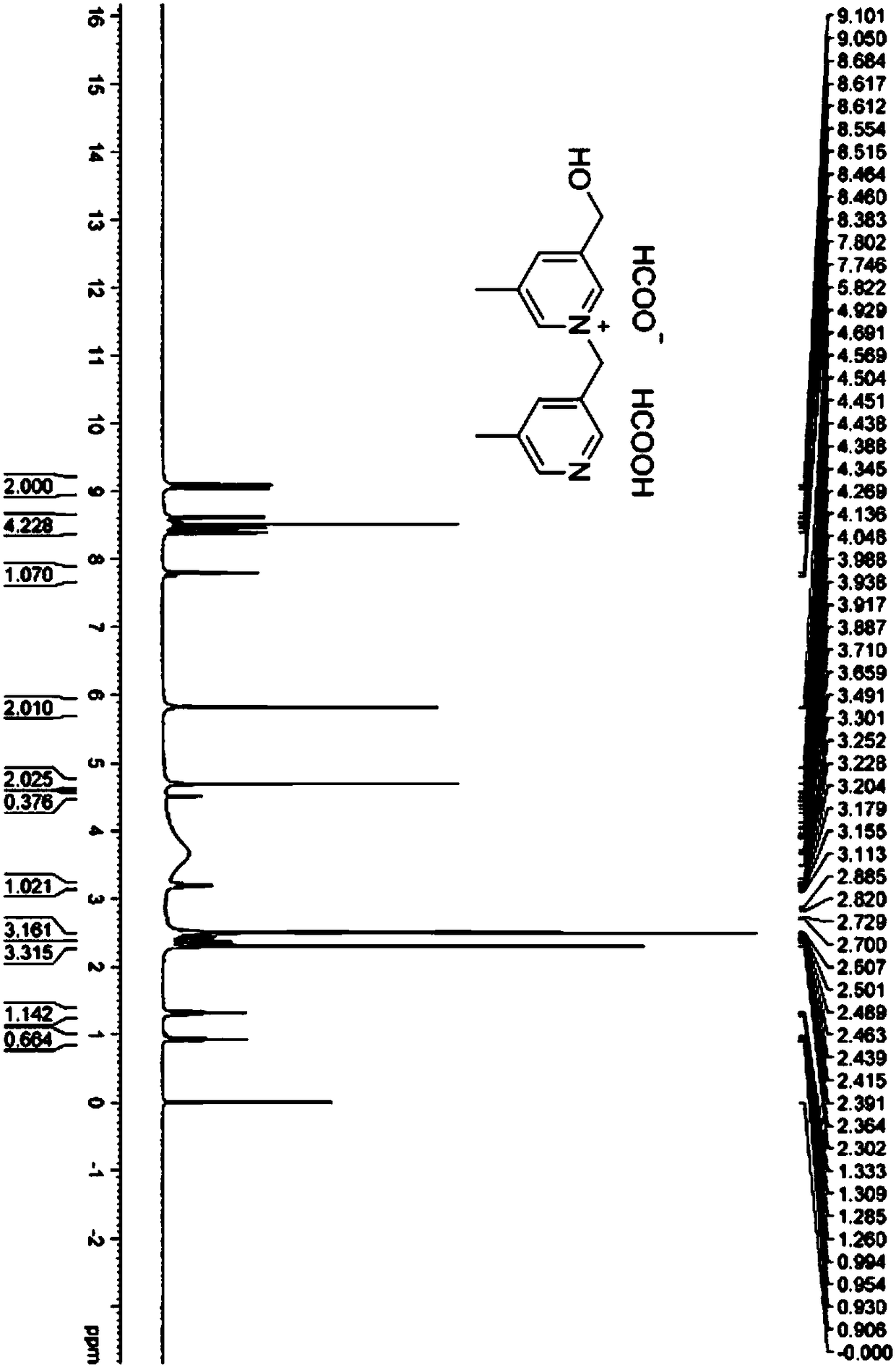
[0075] (1) Substitution reaction: Add 5-methyl-3-pyridinemethanol hydrobromide (25g, 122.51mmol, 1.00equiv.) in a 1L round bottom flask, then add dichloromethane (500mL) and 5 - Methyl-3-bromomethylpyridine hydrobromide (32.7 g, 122.49 mmol, 1.00 equiv.). The above mixture was cooled to 0-5° C. using an ice bath, and triethylamine (37.1 g, 366.64 mmol, 3.00 equiv.) was added dropwise with stirring. After the dropwise addition was completed, a mixture to be reacted was obtained. It was then warmed to room temperature (25 °C) and stirred for 16 h. The reaction system was distilled under reduced pressure to obtain 73 g of crude product. The above-mentioned concentrate was dispersed in 73mL water (1g / mL), and separated and purified by preparative chromatography. The purification conditions were: the chromatographic column was a C18 filler with a pore size of 20um; the mobile phase was water (0.1% formic acid) / acetonitrile, the volume of acetonitrile Scores range from 0 to 10%, a...
Embodiment 2
[0106] The difference from Example 1 is: in the substitution reaction, the molar ratio of raw material A, raw material B and the first acid-binding agent is 1:2:3. The yield of the substituted product was 15 wt%, and the purity was 97 wt%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com