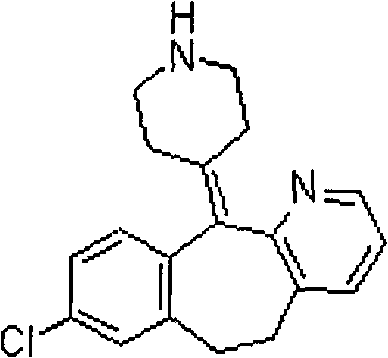
Soft capsule containing desloratadine and preparation method thereof
A technology of desloratadine and soft capsules, which is applied in the field of medicine and can solve the problems of instability of desloratadine when it encounters oxygen, light and humidity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026]
[0027]
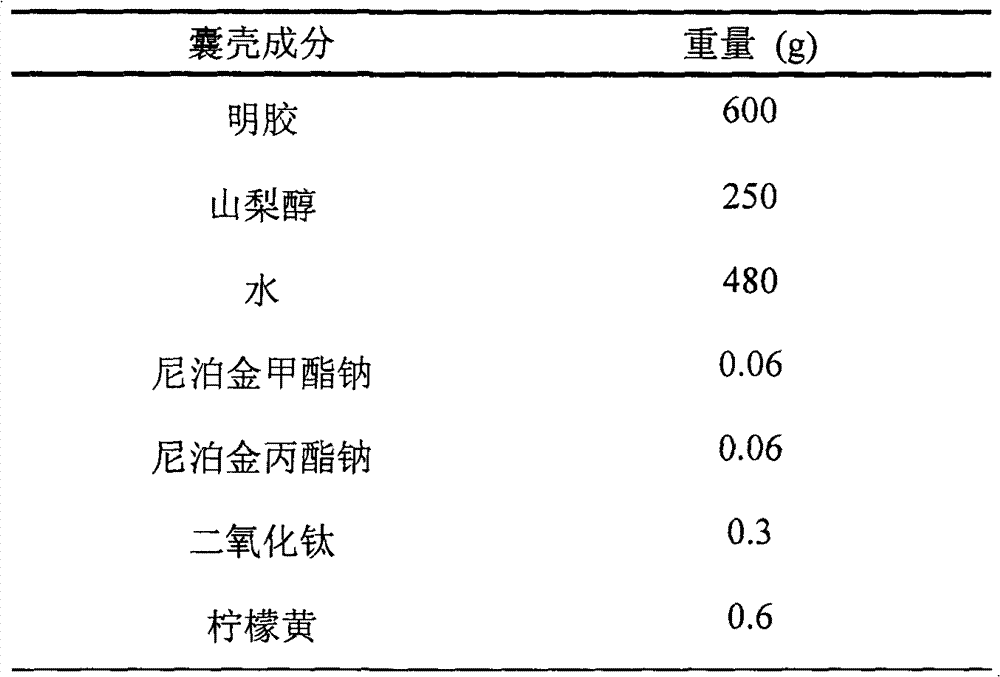
[0028] Capsule material preparation: Weigh the prescribed amount of sorbitol and water, stir evenly, add the prescribed amount of sodium methylparaben, sodium propylparaben, titanium dioxide and tartrazine, stir to mix evenly, quickly add the prescribed amount of gelatin Carry out sol, degas, and set aside.
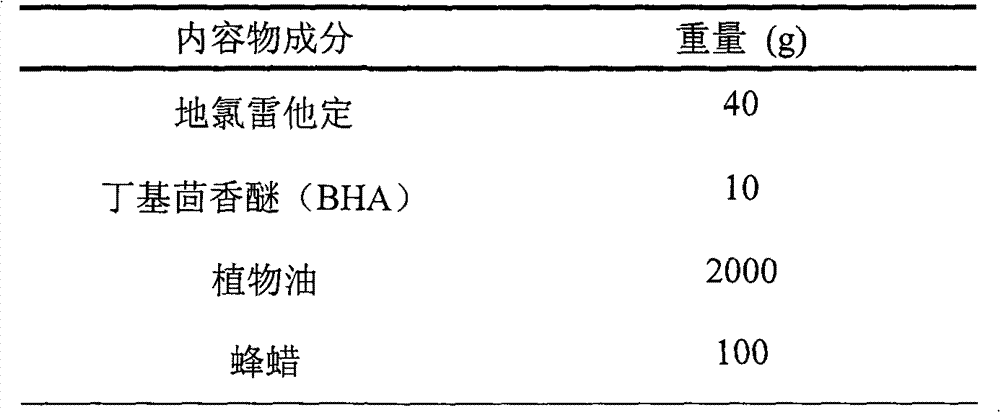
[0029] Preparation of the contents: heat and stir the prescribed amount of beeswax and vegetable oil in the liquid preparation tank through interlayer steam until the beeswax is completely dissolved, and after cooling to room temperature, add the prescribed amount of butyl anisole (BHA) and desloratadine, Mix well and set aside.
[0030] Preparation of soft capsules: pressing pills, washing pills and drying by pressing method.
Embodiment 2
[0032]
[0033]
[0034]
[0035] Capsule material preparation: Weigh the prescribed amount of sorbitol and water, stir evenly, add the prescribed amount of sodium methylparaben, sodium propylparaben, titanium dioxide and tartrazine, stir to mix evenly, quickly add the prescribed amount of gelatin Carry out sol, degas, and set aside.
[0036] Preparation of the contents: heat and stir the prescribed amount of beeswax and vegetable oil in the liquid preparation tank through interlayer steam until the beeswax is completely dissolved, and after cooling to room temperature, add the prescribed amount of butyl anisole (BHA) and desloratadine, Mix well and set aside.
[0037] Preparation of soft capsules: pressing pills, washing pills and drying by pressing method.
Embodiment 3
[0039]
[0040]
[0041] Capsule material preparation: Weigh the prescribed amount of sorbitol and water, stir evenly, add the prescribed amount of sodium methylparaben, sodium propylparaben, titanium dioxide and tartrazine, stir to mix evenly, quickly add the prescribed amount of gelatin Carry out sol, degas, and set aside.
[0042] Preparation of the contents: heat and stir the prescribed amount of beeswax and vegetable oil in the liquid preparation tank through interlayer steam until the beeswax is completely dissolved, and after cooling to room temperature, add the prescribed amount of butyl anisole (BHA) and desloratadine, Mix well and set aside.
[0043] Preparation of soft capsules: pressing pills, washing pills and drying by pressing method.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com