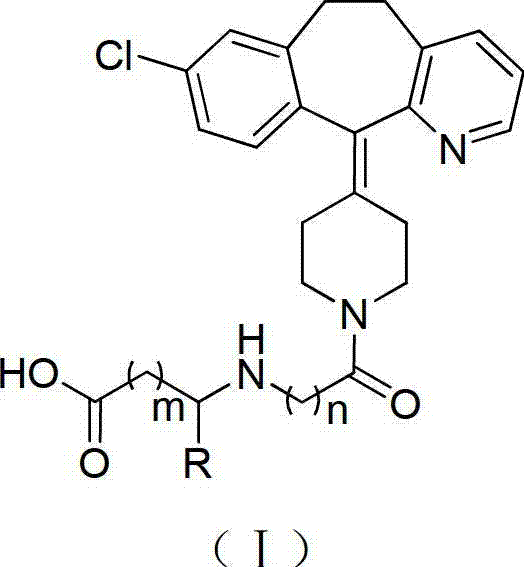
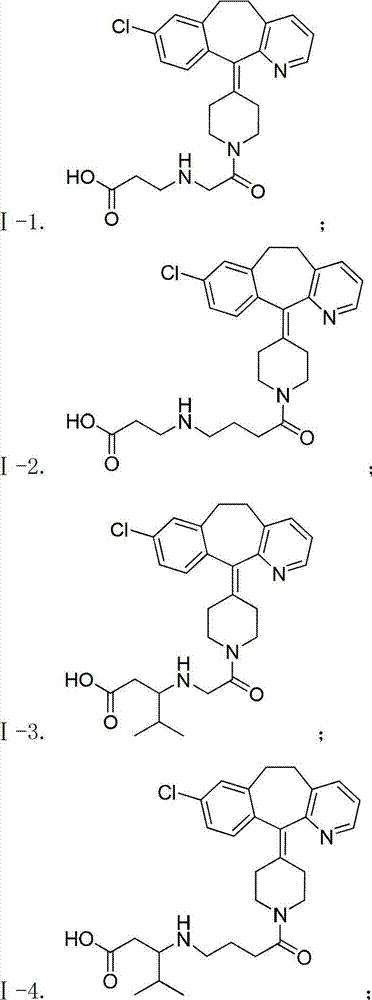
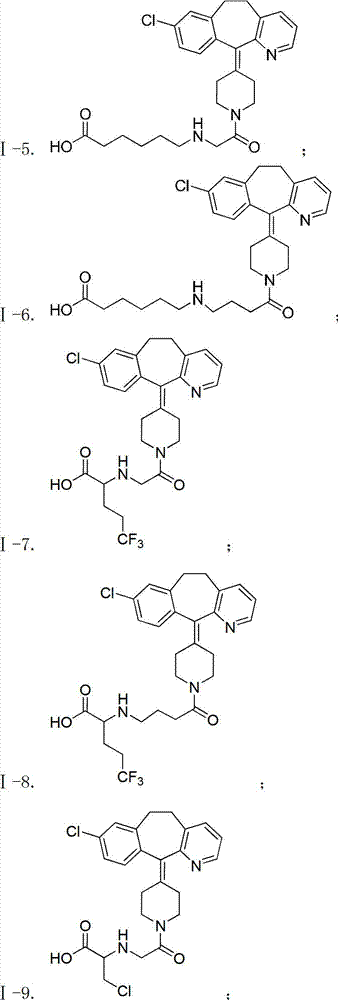
Desloratadine-containing amino acid derivative as well as preparation method and application thereof
A technology of chloromethyl and phenyl, which is applied in the field of anti-tumor compounds and their preparation, and can solve problems such as damage, mutagenesis, and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0051] Preparation of intermediate Ⅲ-1
[0052]
[0053] In the reaction flask equipped with stirring, condenser and thermometer, add 3.10g (10mmol) desloratadine (II) and 30ml of dichloromethane, stir, control the temperature at 0°C, add 2.5g (12mmol) of DCC in batches ) and a catalyst amount of DMAP, after stirring for 1 hour, slowly add 0.76 g (10 mmol) of glycolic acid dropwise, react at 0°C for 3 hours, filter the insoluble matter, and the filtrate is sequentially washed with 10% dilute hydrochloric acid (15ml×3), 10% sodium bicarbonate aqueous solution (15ml×3), washed with saturated brine (15ml×3), filtered off the insoluble matter, dried the filtrate with anhydrous sodium sulfate, evaporated the solvent under reduced pressure, and separated the residue by silica gel column chromatography to obtain a white solid, which was collected Yield 77.5%, purity 95.5% (HPLC normalization method), ESI-MS (m / z): 368.1.
Embodiment 2
[0055] Preparation of Intermediate III-2
[0056]
[0057] In the reaction flask equipped with stirring, condenser and thermometer, add 3.10g (10mmol) desloratadine (II) and 30ml of dichloromethane, stir, control the temperature at 0°C, add 2.5g (12mmol) of DCC in batches ) and a catalytic amount of DMAP, after stirring for 1 hour, slowly add 1.04 g (10 mmol) of 4-hydroxybutyric acid dropwise, react at 0°C for 3 hours, filter the insoluble matter, and wash the filtrate with 10% dilute hydrochloric acid (15ml×3), 10% carbonic acid Wash with sodium hydrogen aqueous solution (15ml×3) and saturated brine (15ml×3), filter off the insoluble matter, dry the filtrate with anhydrous sodium sulfate, evaporate the solvent under reduced pressure, and separate the residue by silica gel column chromatography to obtain white Solid, yield 75.8%, purity 97.5% (HPLC normalization method), ESI-MS (m / z): 396.2.
Embodiment 3
[0059] Preparation of intermediate Ⅳ-1
[0060]
[0061] In a reaction flask equipped with stirring, condenser, and thermometer, add 3.68g (10mmol) of intermediate III-1, 50ml of pyridine and stir to dissolve it. The solution is controlled below -5°C, and 2.28g (12mmol) of Toluenesulfonyl chloride was reacted at 0°C for 10 hours. TLC showed that the reaction was complete. Pour the reaction solution into cold water, and a solid precipitated out. After filtration, the filter cake was washed with saturated brine (50ml×3), and dried in vacuo to obtain a white solid. Yield 92.2%, purity 98.0% (HPLC normalization method), ESI-MS (m / z): 522.1.
[0062] Reference Example 4:
[0063] Preparation of intermediate IV-2
[0064]
[0065] Add 3.96g (10mmol) of intermediate III-2, 50ml of N,N-dimethylformamide and 2.52g (25mmol) of triethylamine into a reaction flask equipped with stirring, condenser, and thermometer, stir to dissolve , the solution is kept below -5°C, add 2.28g (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com