Method of treatment and device for the improved bioavailability of leukotriene receptor antagonists
a technology of leukotriene receptor and bioavailability, which is applied in the direction of heterocyclic compound active ingredients, pharmaceutical delivery mechanisms, inorganic non-active ingredients, etc., can solve the problems of inconsistent bioavailability, inability to fully absorb leukotriene, so as to improve the bioavailability of leukotriene antagonists and improve the bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
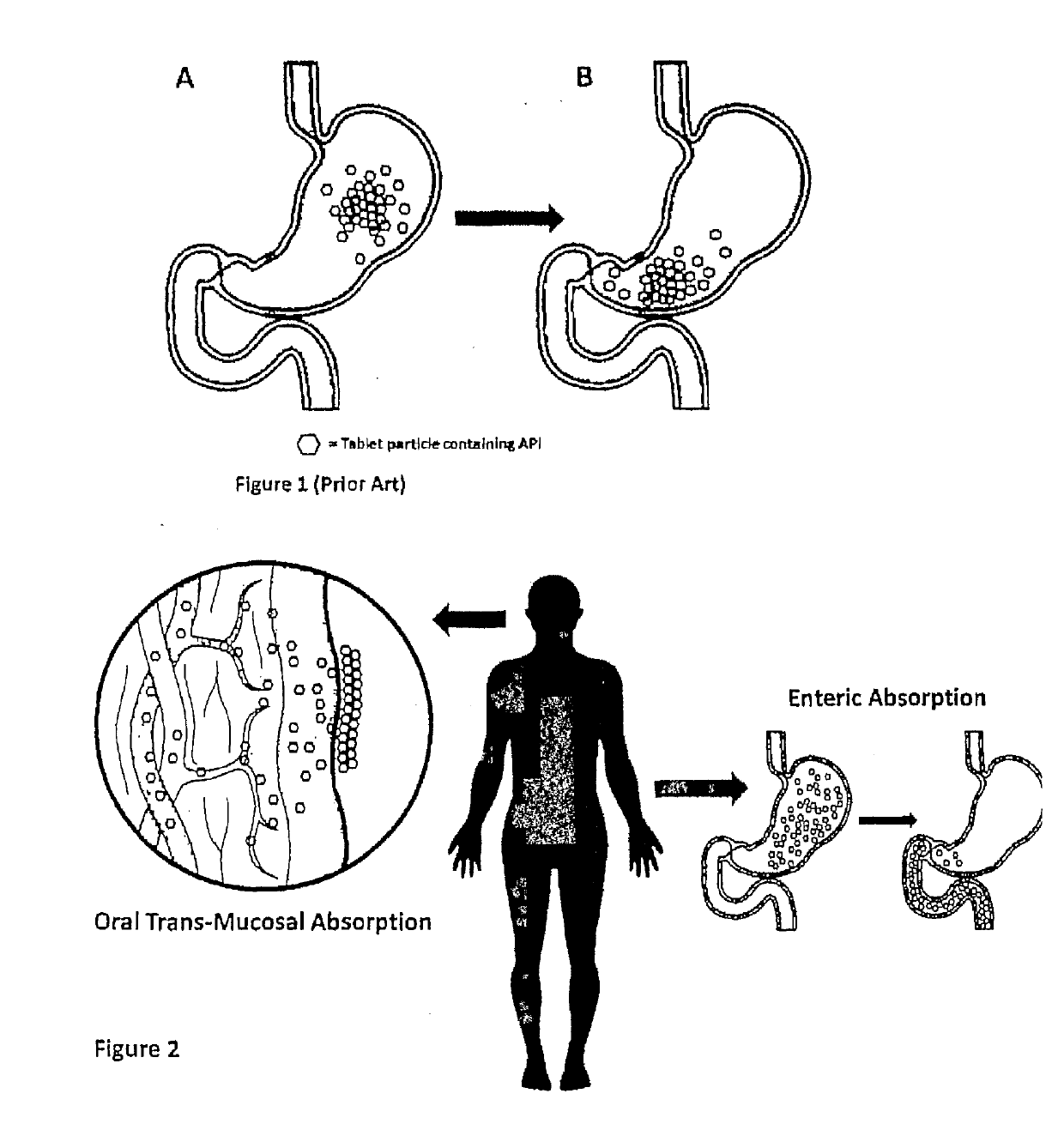
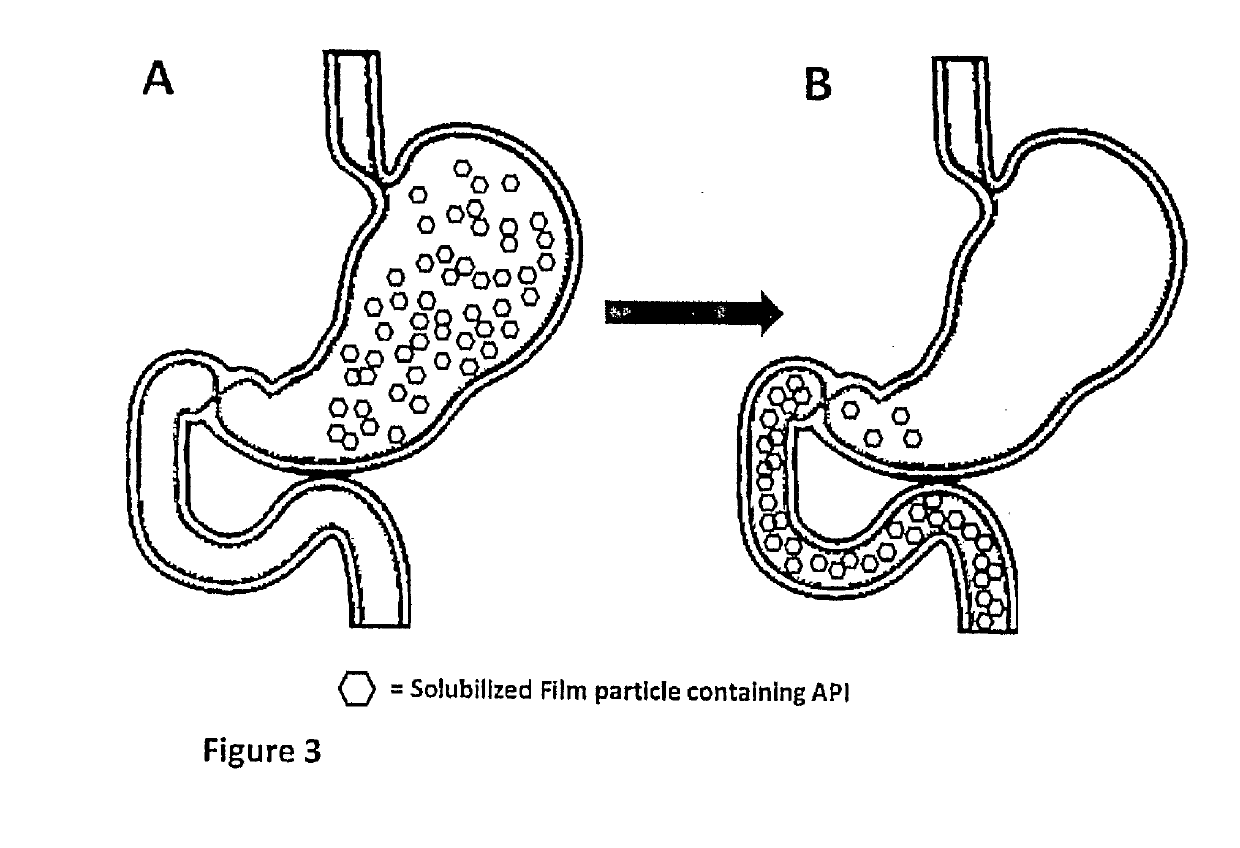
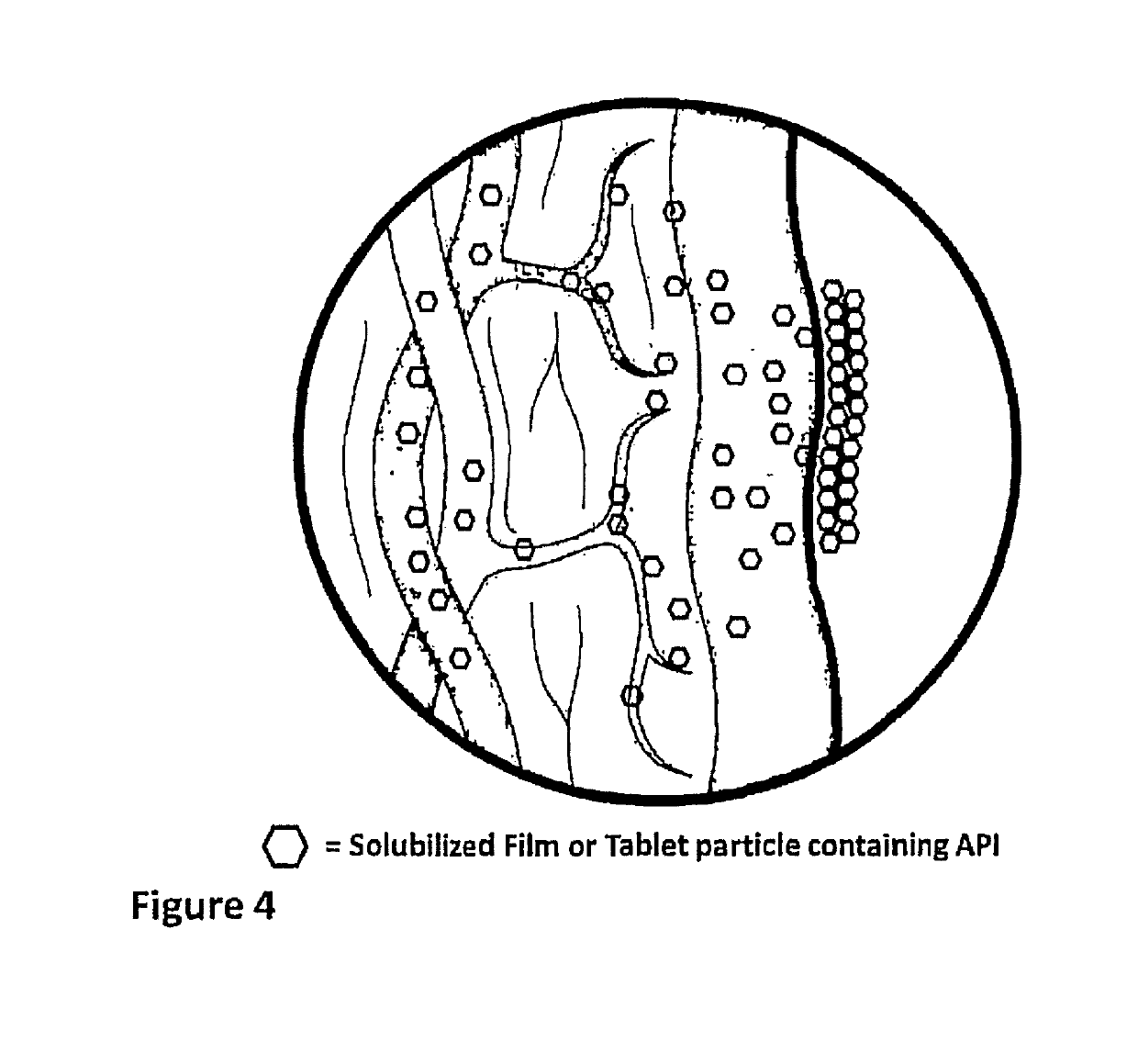
[0055]In accordance with certain aspects of this disclosure, methods of administration and devices for the improved bioavailability of leukotriene inhibitors are provided. These methods and devices involve an oral dosage form designed to deliver leukotriene inhibitors such as Montelukast, to the mouth and stomach in the form of an amorphous precipitate suspended in an aqueous medium (e.g., saliva and / or gastric fluids).
[0056]In accordance with certain aspects of this disclosure, methods for treating neurodegenerative diseases and / or other conditions that are at least partially induced by leukotrienes are provided. These methods include enteral delivery or a combination of transmucosal, sublingual or both transmucosal and sublingual, along with enteral delivery of Montelukast. The Montelukast is incorporated into a film layer in an amount that is safe and effective to reduce leukotriene induced neuroinflammation in patients.
[0057]Neurodegenerative diseases that can be treated in acco...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com