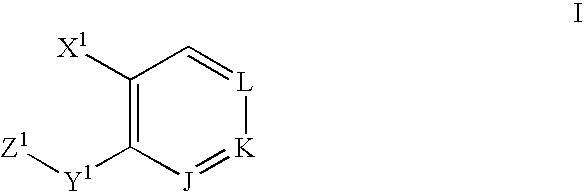
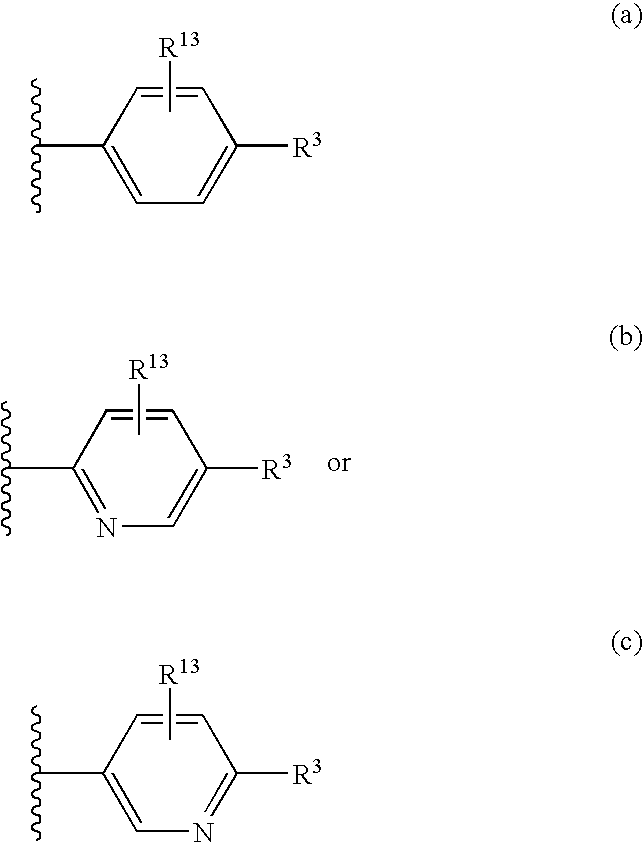
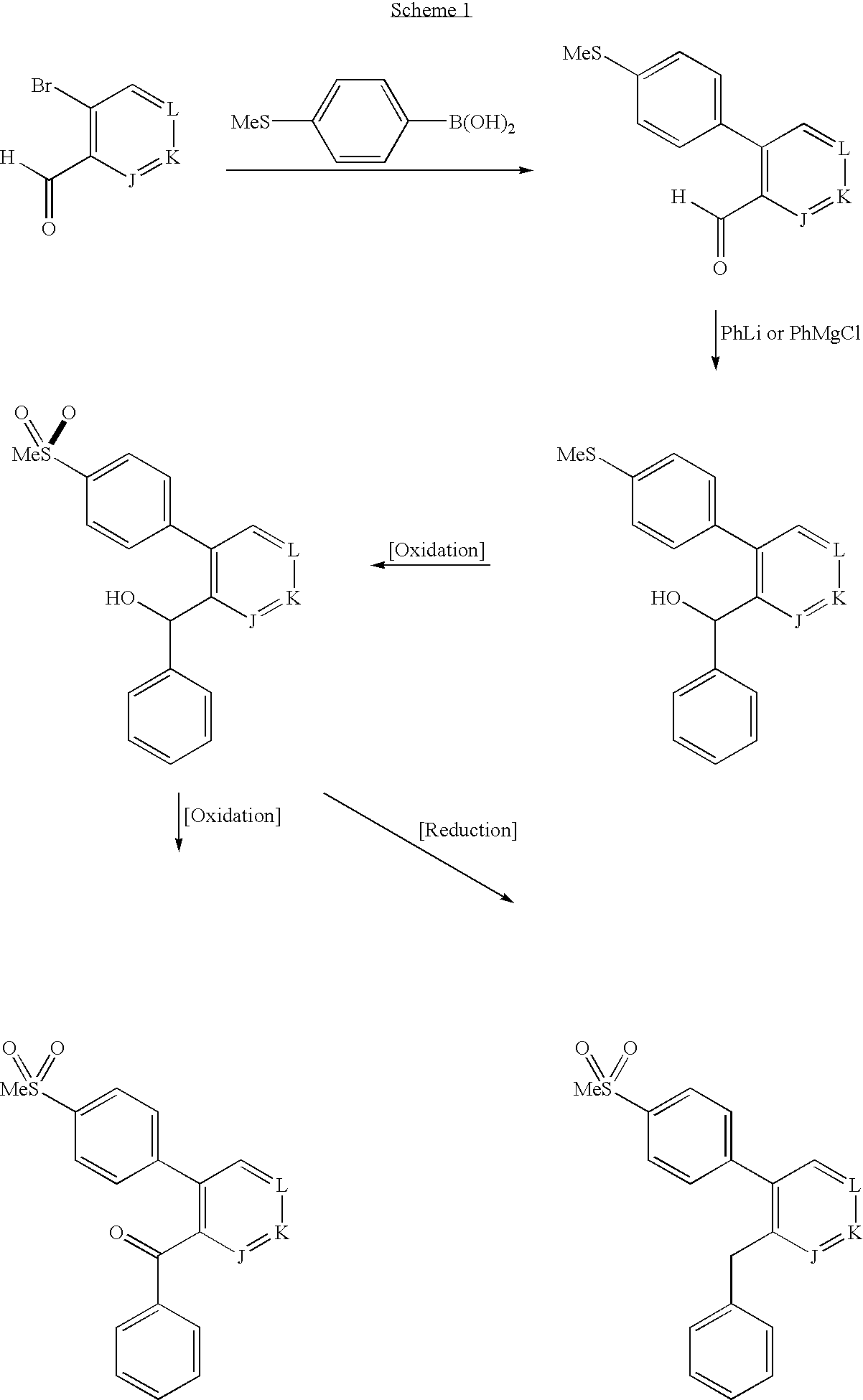
Substituted aryl compounds as novel cyclooxygenase-2 selective inhibitors, compositions and methods of use
a selective inhibitor and cyclooxygenase technology, applied in the field of substituted aryl compounds, can solve the problems of undesirable side effects, gastrointestinal ulceration and renal toxicity, and the repeated use of nsaids has been associated with adverse effects, so as to improve the cardiovascular profile of cox-2 selective inhibitors, anti-inflammatory properties, and unexpected potential for wound healing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
1-(6-(Cyclohexylmethyl)(2-H-benzo(3,4-d)1,3-dioxolen-5-yl))-4-(methylsulfonyl)benzene
1 a. 6-Bromo-2H-benzo(d) 1,3-dioxolene-5-carbaldehyde
The title compound was synthesized as described in the literature (Khanapure, S. P. and Biehl, E. R. J. Org. Chem. 1990, 55, 1471). Treatment of piperonal (40 g) with bromine (40 mL) in acetic acid (500 mL) and carbon disulfide (50 mL) containing a catalytic amount of iodine at room temperature, overnight, gave the title compound (46 g, 70% yield), mp 128-130° C. 1H NMR (300 MHz, CDCl3) δ10.17 (s, 1H, 7.33 (s, 1H), 7.03 (s, 1H), 6.06 (s, 2H), 13C NMR (75 MHz, CDCl3) δ190.3, 153.3, 148.1, 128.0, 121.5, 113.2, 108.1, 102.7; mass spectrum (API-TIS) m / z 229 (Br 79) and 231 (Br 81) (M+H) LRMS (APIMS) m / z 229 (M+H)+ and 231 ((M+H)+2)+.
1b. 6-(4-Methylthiophenyl)-2H-benzo(d) 1,3-dioxolene-5-carbaldehyde
The product of Example 1a (1.15 g, 5 mmol) and 4-(methylthio)benzeneboronic acid (840 mg, 5 mmol) were dissolved in toluene (75 mL) and sodium carb...
example 2
Cyclohexyl(6-(4-(methylsulfonyl)phenyl)(2H-benzo(d)1,3-dioxolan-5-yl) ketone
2a. Cyclohexyl(6-(4-methylthiophenyl)(2H-benzo(d) 1,3-dioxolan-5-yl))methan-1-ol
The product of Example 1b (272 mg, 1 mmol) was dissolved in anhydrous THF (10 mL). The solution was cooled to 0° C. and cyclohexyl magnesium bromide (2 M in THF, 2 m / L, 2 mmol) was added drop-wise under nitrogen atmosphere. The reaction mixture was stirred at 0° C. for 30 minutes and then at room temperature for 2 hours. The reaction was quenched with saturated aqueous ammonium chloride, acidified with 1 N HCl and then extracted with ethyl acetate (2×50 mL). The combined organic extracts were washed with water (1×25 mL), brine (1×25 mL), dried over sodium sulfate, filtered and the filtrate was evaporated under reduced pressure to give the crude product. Purification by silica gel column chromatography using 20% ethyl acetate in hexane gave the title compound as a white solid (331 mg, 93% yield), mp 110-112 0° C. 1H NMR (CDCl3...
example 3
6-(4-(Methylsulfonyl)phenyl)(2H-benzo(d)1,3-dioxolan-5-yl)phenyl ketone
3a. (6-(4-Methylthiophenyl)(2H-benzo(d) 1,3-dioxolan-5-yl))phenylmethan-1-ol
The product of Example 1b (2.72 g, 10 mmol) was dissolved in anhydrous THF (100 mL). The solution was cooled to 0° C. and phenyl magnesium chloride (2 M in THF) (12 mL, 24 mmol) was added drop-wise under a nitrogen atmosphere. The reaction mixture was stirred at 0° C. for 30 minutes and then at room temperature for 2 hours. The reaction was quenched with saturated aqueous ammonium chloride, acidified with 1 N HCl and the THF layer was separated. The aqueous layer was extracted with ethyl acetate. The combined organic extracts were dried over sodium sulfate, filtered and the filtrate was evaporated under reduced pressure to give the crude product. Purification by silica gel column chromatography using 20% ethyl acetate in hexane as the eluant gave the title compound as a white solid (2.3 g, 66% yield), mp 96-99° C. 1H NMR (CDCl3) δ 7.2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| swelling | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| Platelet aggregation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com