Conjugates of insulin-like growth factor-1 and poly(ethylene glycol)
a technology of insulinlike growth factor and conjugate, which is applied in the field of conjugates of insulinlike growth factor and poly(ethylene glycol) can solve the problems of unsuitability for commercial use of many nhs-pegylated proteins and complex regulation of igf-i function, and achieve the effect of superior properties in regard to therapeutic applicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Chromatographical Separation of monoPEG-IGF-I Positional Isomers
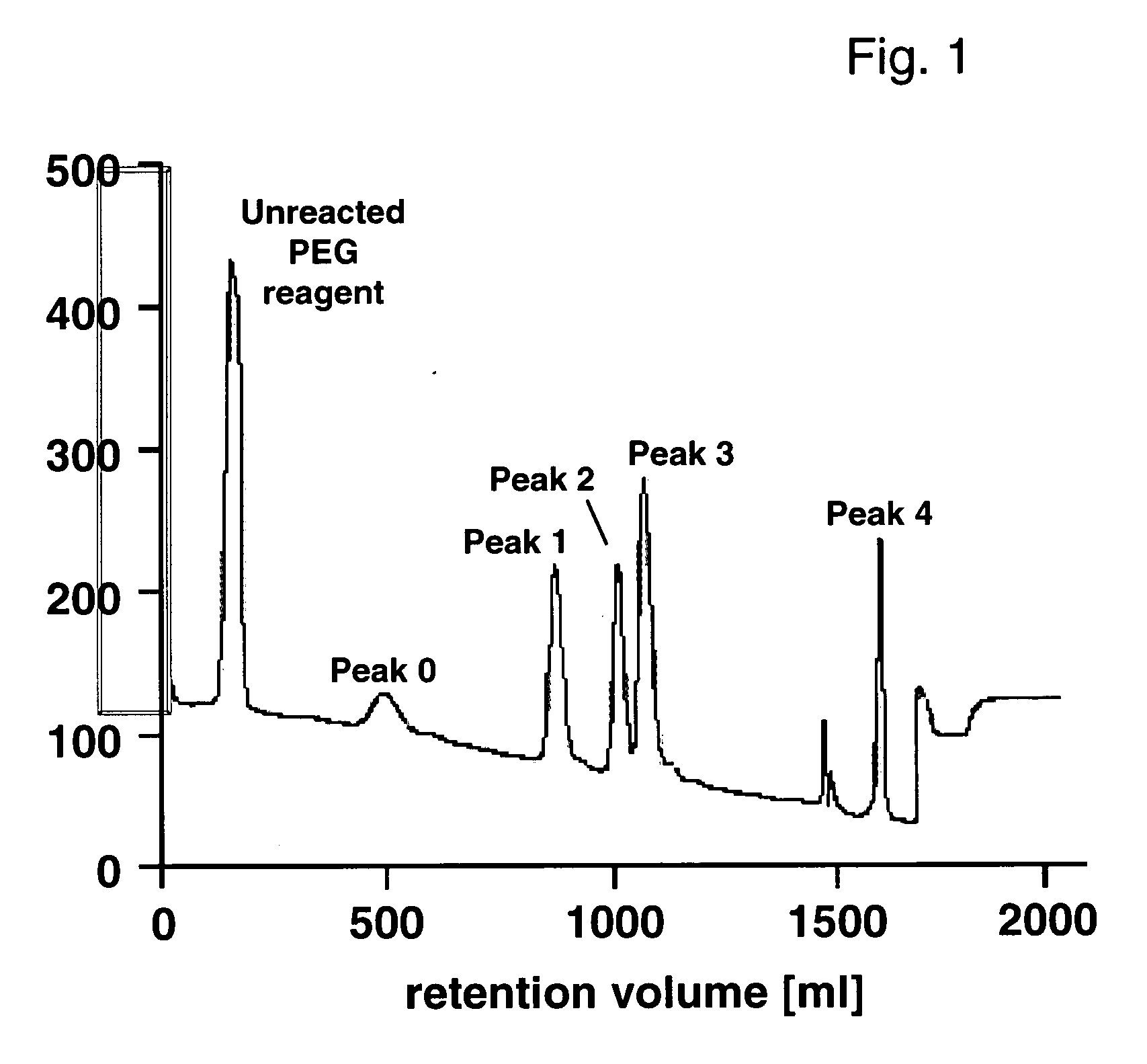
[0088] IGF-I contains 4 amino groups as potential PEGylation sites and 4 possible monoPEGylated IGF-I (monoPEG-IGF-I) isomers were expected. Further derivates were expected to be oligoPEGylated depending on the reaction conditions. A strong-cation high pressure liquid chromatography method (IEC-HPLC) was developed for the separation of PEG-IGF-I or -Des(1-3)-IGF-I isomers based on their local charge differences. A preparative elution profile of 5 mg PEG-IGF-I is shown in FIG. 1. The result of this method was a separation into 5 major peaks, 3 peaks with baseline separation and 2 with partial separation. The decrease of the baseline absorption towards the end of the chromatogram suggests no additional monoPEGylated IGF-I species eluting at higher retention time. An additional peak appears between peak 3 and 4 resulting from the switch to the modified buffer B with different pH. Analytical IEC-HPLC was used to estimate t...
example 2
SDS-PAGE Analysis of monoPEG-IGF-I Isomers
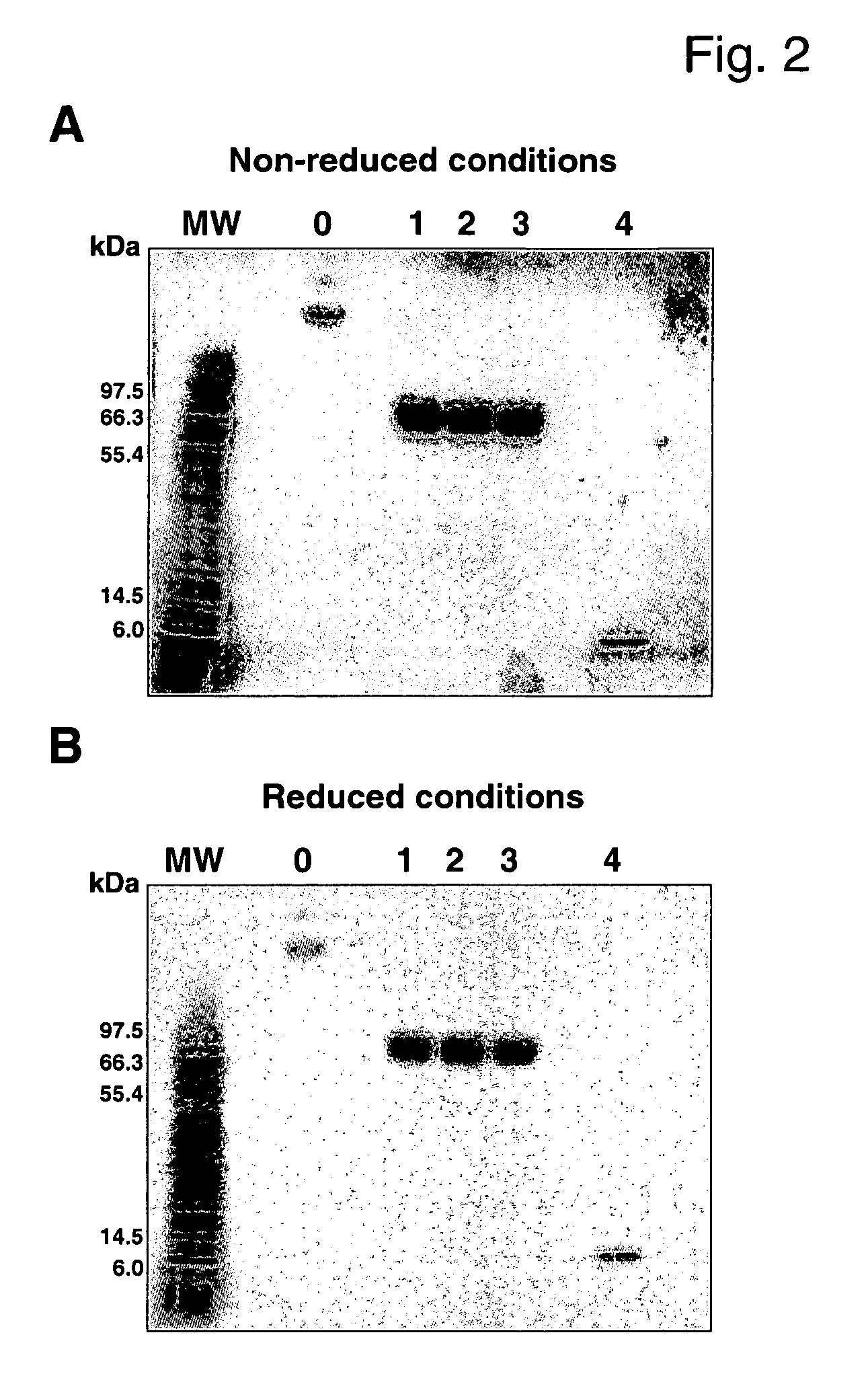
[0089] SDS-PAGE was performed both under non-reducing and reducing conditions to evaluate potential unwanted cross-linking of different IGF-I molecules through intermolecular disulfide bridges. Both conditions yielded similar results indicating that no significant amount of the protein was abnormally cross-linked (FIG. 2). The SDS-PAGE analysis showed that peak 0 had an apparent molecular weight of >100 kDa whereas the major detected bands were peaks 1-3 at ˜70 kDa; additionally, peak 4 was detected at ˜7 kDa, a size expected for unPEGylated IGF-I (FIG. 2). From this running profile and the retention times obtained in the HPLC we concluded that peak 0 consists most probably of di- and oligoPEG-IGF-I. In contrast, peaks 1-3 were designated as 3 different monoPEG-IGF-I isomers. There was a discrepancy between the expected molecular weight for monoPEG-IGF-I (51.6 kDa) and the apparent size of ˜70 kDa; however, the observed higher apparent mole...
example 3
Analysis of monoPEG-IGF-I Isomers
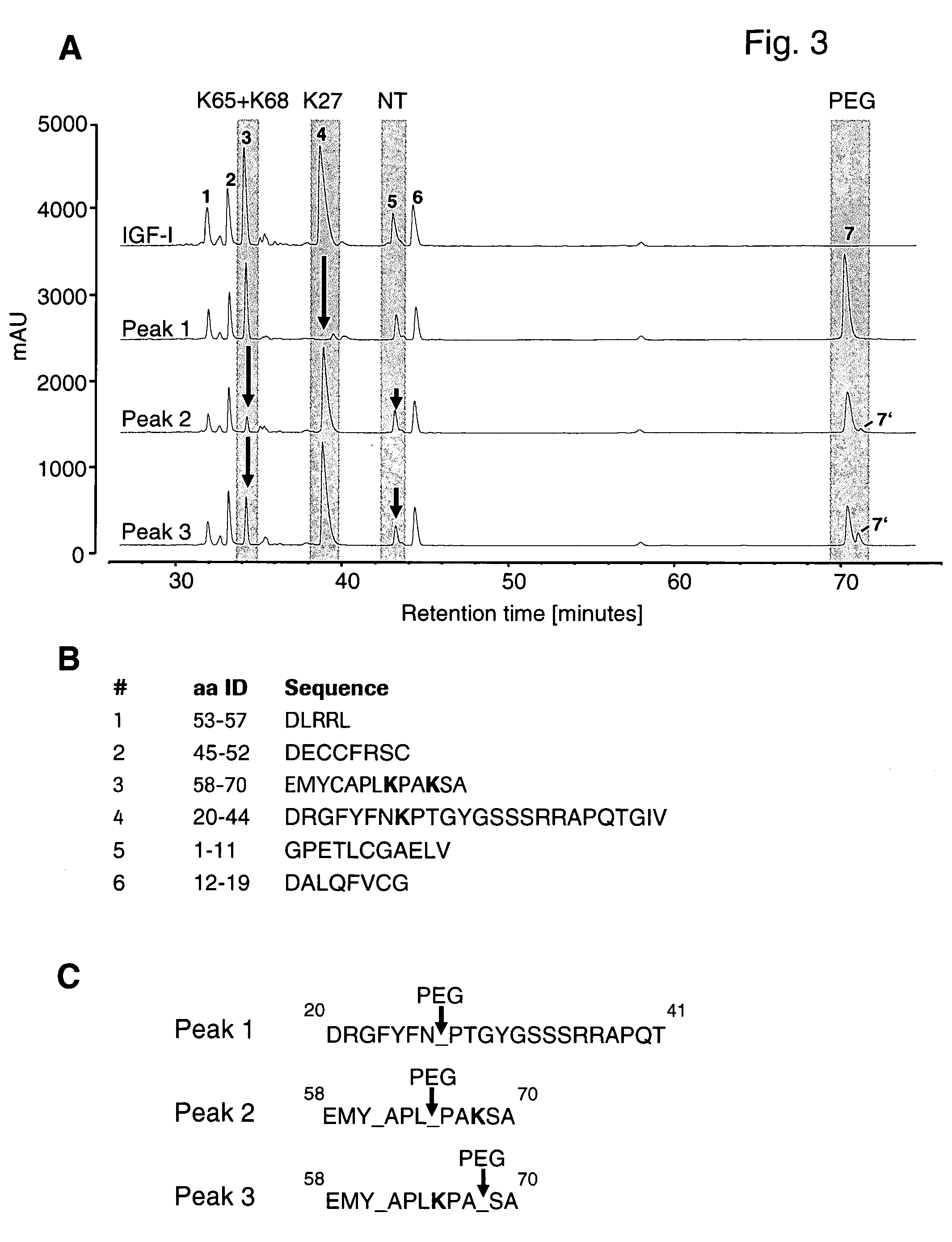
[0092] Cleavage of rhIGF-I with Asp-N delivered 6 independent peptide fragments that were separated by HPLC between 30 and 45 minutes retention time (FIG. 3A,upper panel). After cleavage of the purified peaks 1 to 3 (FIG. 3A, lower panels), different distributions of the 6 fragments and the occurrence of an additional fragment (fragment 7) was observed eluting at 70 minutes retention time. For peak 1, specifically peptide fragment 4 was diminished with a concomitant increase of fragment 7. For peak 2, we observed a clear decrease in fragment 3 and a slight decrease in fragment 5 and the appearance of a major and a minor PEG fragment (7 and 7′). Similarly, HPLC analysis of peak 3 yielded a clear decrease in fragments 3 and 5 with the concomitant occurrence of fragments 7 and 7′. FIG. 11B shows the peptide sequences of the respective fragments as obtained by Asp-N cleavage of rhlGF-I. Using Edman N-terminal peptide degradation we analyzed the peptide...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com