Application of dihydromyricetin in preparation of medicines for inhibiting adriamycin cardiotoxicity
A technology for dihydromyricetin and cardiotoxicity, which is used in drug combinations, antitumor drugs, pharmaceutical formulations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Rat primary cardiomyocytes were inoculated in a 96-well plate, and a blank control group, adriamycin (ADR) single use group and dihydromyricetin (DMY) pretreatment group were set up. Each group set 5 ADR concentration gradients, 2-fold dilution. Each concentration was replicated in triplicate. After the cells adhered to the wall, the dihydromyricetin (DMY) pretreatment group was added with DMY and cultured in the incubator for 24 hours. Then add different concentrations of ADR, continue to cultivate in the incubator for 48 hours, add 5 mg / ml MTT solution, and incubate for another 4 hours, then aspirate the liquid and add DMSO to completely dissolve formazan, then use a microplate reader to measure its concentration at 570 Absorbance was measured at nm. The results showed that after 0.5, 1, 2, 4 and 8 mM ADR, the growth of rat primary cardiomyocytes was inhibited in a dose-dependent manner, while DMY 25 and 50 mM pretreatment showed a significant reversal of the inhibi...
Embodiment 2
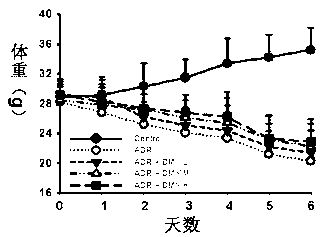
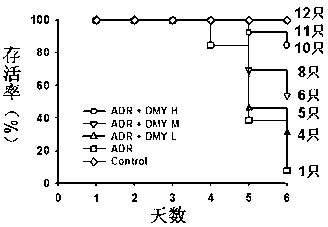
[0021] Forty-eight ICR male mice were randomly divided into 4 groups, including blank control group, doxorubicin model group and three DMY pretreatment groups with 12 each. The blank control group was given the same amount of normal saline; the doxorubicin model group was intragastrically administered with normal saline for four consecutive days, and 20 mg / kg doxorubicin was injected intraperitoneally on the fourth day. The DMY pretreatment group were given 125 mg / kg, 250 mg / kg, and 500 mg / kg DMY, respectively, for four consecutive days by intragastric administration, and 20 mg / kg doxorubicin was injected intraperitoneally 30 minutes after administration on the fourth day. The mice were weighed every day and the time of death was recorded. The results showed that the survival time of the mice in the 500 mg / kg dihydromyricetin pretreatment group was significantly longer than that of the model group, and the weight loss was significantly relieved than that of the model group. S...
Embodiment 3
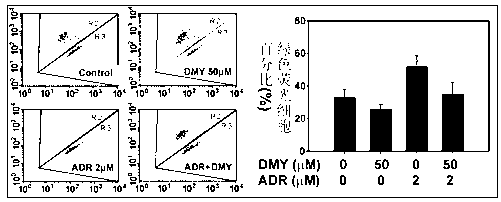
[0023] Rat primary cardiomyocytes were pretreated with dihydromyricetin, and then collected 48 hours after ADR. JC-1 is a cationic dye that can accumulate in mitochondria in cells to form polymers and emit red fluorescence; when the mitochondrial membrane potential is damaged, JC-1 cannot be aggregated into mitochondria, but as a monomer The form of JC-1 exists in the cytoplasm, and at this time JC-1 emits green fluorescence. According to this characteristic, we used JC-1 staining combined with flow cytometry to measure the changes of mitochondrial membrane potential. The result is as image 3 As shown, 32.6±5.8% of the cardiomyocytes in the solvent control group showed green fluorescence, and after 2 mM ADR for 48 hours, 52.6±6.2% of the cardiomyocytes showed green fluorescence (that is, the membrane potential decreased), while after pretreatment with 50 mM dihydromyricetin , 35.5±7.8% of cardiomyocytes showed green fluorescence, and the mitochondrial membrane potential in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com