Application of glycyrrhizic acid in preparation of sunitinib malate cardiotoxicity reduction drug
A technology of sunitinib maleate and cardiotoxicity, applied in the field of application of glycyrrhizic acid in the preparation of medicines for alleviating the cardiotoxicity of sunitinib maleate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
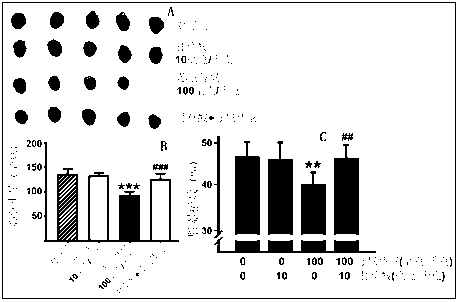
[0018] Rat primary cardiomyocytes were inoculated in a 96-well plate, and a blank control group, a Sunitinib maleate (Sunitinib) monotherapy group and a glycyrrhizic acid (GA) pretreatment group were set up. Each group set up 5 concentration gradients, 2-fold dilution. Each concentration was replicated in triplicate. After the cells adhered to the wall, glycyrrhizic acid (GA) was added and cultured in the incubator for 24 hours. Then add different concentrations of sunitinib maleate (Sunitinb), continue to cultivate in the incubator for 48 hours, add 5 mg / ml MTT solution, incubate for another 4 hours, then aspirate the liquid and add DMSO to completely dissolve formazan The absorbance was measured at 570 nm with a microplate reader. As shown in Table 1, after the action of 0.3125, 0.625, 1.25, 2.5, 5 and 10 mM sunitinib maleate (Sunitinib), the growth of rat primary cardiomyocytes was inhibited in a dose-dependent manner, while glycyrrhizic acid (GA) After 25 and 50 mM pret...
Embodiment 2
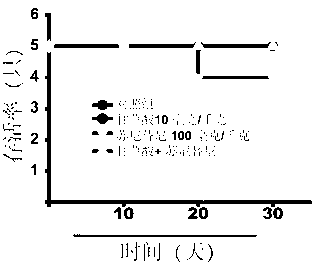
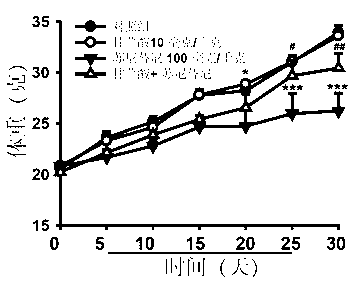
[0021] Forty ICR male mice were randomly divided into 4 groups, 10 in each of the blank control group, Sunitinib model group and Glycyrrhizic acid (GA) pretreatment group. The blank control group was given the same amount of normal saline; the glycyrrhizic acid (GA) single use group was given 10 mg / kg glycyrrhizic acid; 100 mg / kg Sunitinib maleate (Sunitinib). The glycyrrhizic acid (GA) pretreatment group was given 10 mg / kg glycyrrhizic acid, orally administered for four consecutive days, and 100 mg / kg Sunitinib maleate (Sunitinib) by intragastric administration on the fourth day. The mice were weighed every day and the survival of the mice was recorded, and the administration was continued for 30 days. Twenty-four hours after the last administration, the electrocardiogram (Lead II) of the mice in each group was traced in a quiet environment, and the QRS complex wave and S-T wave of the mice were calculated. Afterwards, the mice in each group were removed from the eyes to co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com