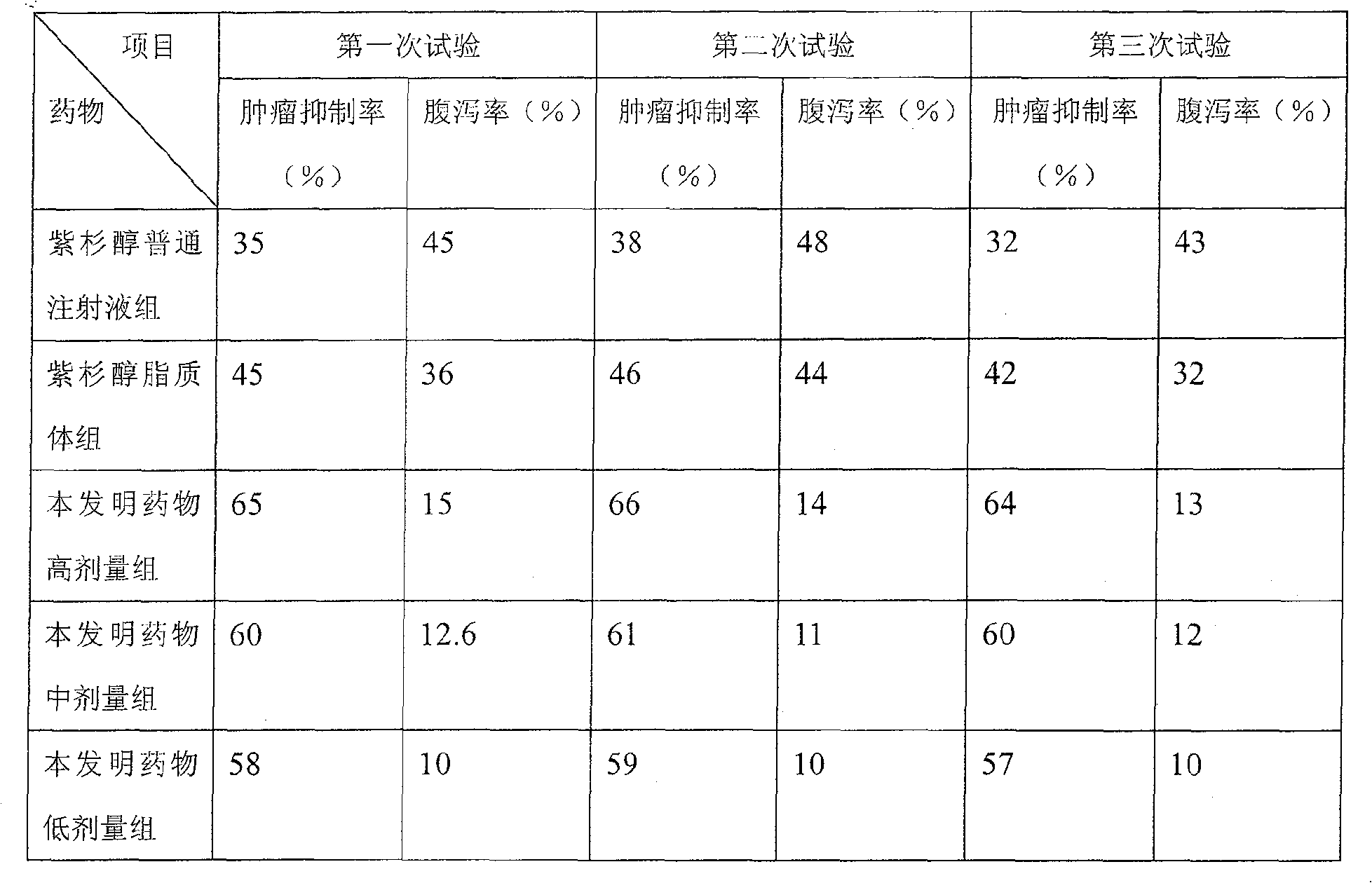Paclitaxel/multialkene paclitaxel liposome composite medicine and preparation method thereof
A liposome and drug technology, applied in the directions of liposome delivery, drug combination, pharmaceutical formulation, etc., can solve problems such as side effects, neutropenia, tumor cell death, etc. Exponentially high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The formula that present embodiment adopts is as follows:
[0035] 300g of a mixture of hydrogenated soybean lecithin and sheep brain lecithin in a weight ratio of 2:1;
[0036] 200g of a mixture of cholesterol, stearylamide, and β-sitosterol in a weight ratio of 1:1:0.1;
[0037] 100g of a mixture of tiopronin and vitamin E in a weight ratio of 4:1;
[0038] 150g of any ratio mixture of Polyethylene Glycol-4000 and Polyethylene Glycol 6000;
[0039] Vitamin C 150g;
[0040] 4000g of mixture of tert-butanol and absolute ethanol in a weight ratio of 1.5-2.2:0.01-0.05;
[0041] A mixture of paclitaxel and docetaxel in any ratio 20g;
[0042] Diphenhydramine, cimetidine weight ratio 1: 6 mixture 350g;
[0043] Mix in 12000g of 0.01M, pH6.0-7.5 phosphate buffer.
[0044] The present invention also provides a preparation method of the above-mentioned combination medicine, which is characterized in that it comprises the following steps:
[0045] (1) Add the arbitrary r...
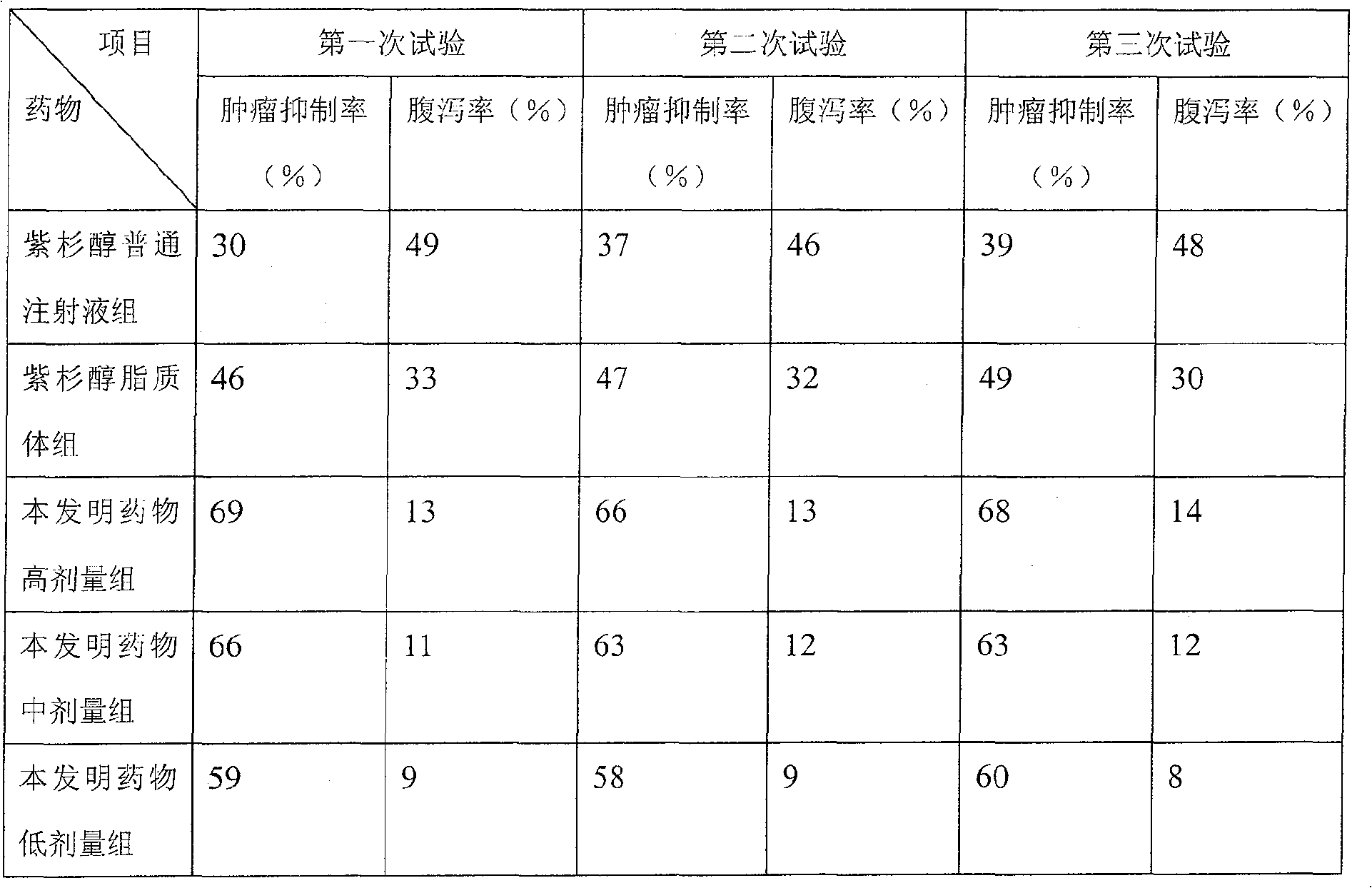
Embodiment 2
[0069] The formula adopted in the present embodiment is as follows (consumption: gram):
[0070] A mixture of hydrogenated soybean lecithin and sheep brain lecithin in a weight ratio of 2:1 550;
[0071] Cholesterol, stearylamide, β-sitosterol mixture with a weight ratio of 1:1:0.1 400;
[0072] The mixture of tiopronin and vitamin E in a weight ratio of 4:1 200;
[0073] Any ratio mixture of polyethylene glycol-4000 and polyethylene glycol 6000 200;
[0074] Vitamin C 180;
[0075] A mixture of tert-butanol and absolute ethanol in a weight ratio of 1.5-2.2:0.01-0.05 7000;
[0076] Mixture of paclitaxel and docetaxel in any ratio 40;
[0077] A mixture of diphenhydramine and cimetidine in a weight ratio of 1:6 500;
[0078] Mix in 12000g of 0.01M, pH6.0-7.5 phosphate buffer.
[0079] The present invention also provides a preparation method of the above-mentioned combination medicine, which is characterized in that it comprises the following steps:
[0080] (1) Add the a...
Embodiment 3
[0104] The formula adopted in the present embodiment is as follows (consumption: gram):
[0105] A mixture of hydrogenated soybean lecithin and sheep brain lecithin in a weight ratio of 2:1 400;
[0106] Cholesterol, stearylamide, β-sitosterol mixture with a weight ratio of 1:1:0.1 300;
[0107] The mixture of tiopronin and vitamin E in a weight ratio of 4:1 150;
[0108] Any ratio mixture of polyethylene glycol-4000 and polyethylene glycol 6000 180;
[0109] Vitamin C 165;
[0110] A mixture of tert-butanol and absolute ethanol in a weight ratio of 1.5-2.2:0.01-0.05 6000;
[0111] Mixture of paclitaxel and docetaxel in any ratio 32;
[0112] A mixture of diphenhydramine and cimetidine in a weight ratio of 1:6 420;
[0113] Mix in 12000g of 0.01M, pH6.0-7.5 phosphate buffer.
[0114] The present invention also provides a preparation method of the above-mentioned combination medicine, which is characterized in that it comprises the following steps:
[0115] (1) Add the a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com