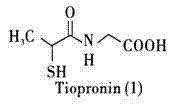
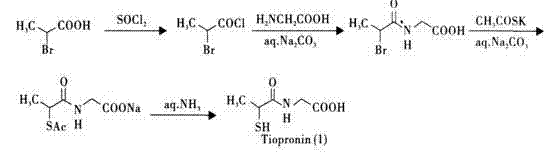
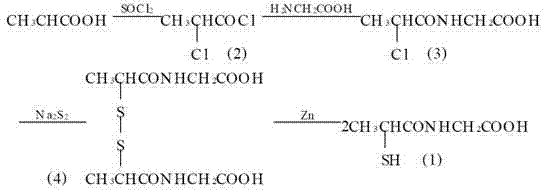
Method for preparing tiopronin
A technology of tiopronin and sodium disulfide, applied in the field of medicine, can solve problems such as low yield of tiopronin, unfavorable treatment of three wastes, and increased reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0034] 1. Preparation of α-chloropropionyl chloride
[0035] Add 108.6 g (1 mol) of α-chloropropionic acid and 178.4 g (1.5 mol) of thionyl chloride into a 100 mL round-bottomed flask, reflux for 5 h, collect the distillate with bp 109~111°C to obtain 120.1 g of colorless liquid , the yield is 94.5%.
[0036] 2. Preparation of α-chloropropionylglycine
[0037] Add 37.4 g (0.5 mol) of glycine, 53.0 g (0.5 mol) of anhydrous sodium carbonate and 500ml of water into a 1000 mL four-necked flask, and stir to dissolve. Cool in an ice-salt bath, and add α-chloropropane dropwise under vigorous stirring Acyl chloride 63.3 g (0.5 mol), continue to stir for 3 h after addition, acidify with hydrochloric acid to pH = 1, extract with ethyl acetate, dry over anhydrous sodium sulfate, filter, concentrate the filtrate until crystals precipitate, place, filter The precipitated crystals were dried to obtain 56.1 g of small colorless needle-like crystals, with a yield of 68%, mp 104~105°C.
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com