Pronin medicinal composition and its preparing method
A technology of tiopronin and medicine, which is applied in the field of tiopronin combination medicine and its preparation, can solve the problems of reduced drug efficacy, toxic and side effects, etc., and achieve the effects of improving curative effect, reducing the occurrence of proteinuria, and reducing the amount of distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
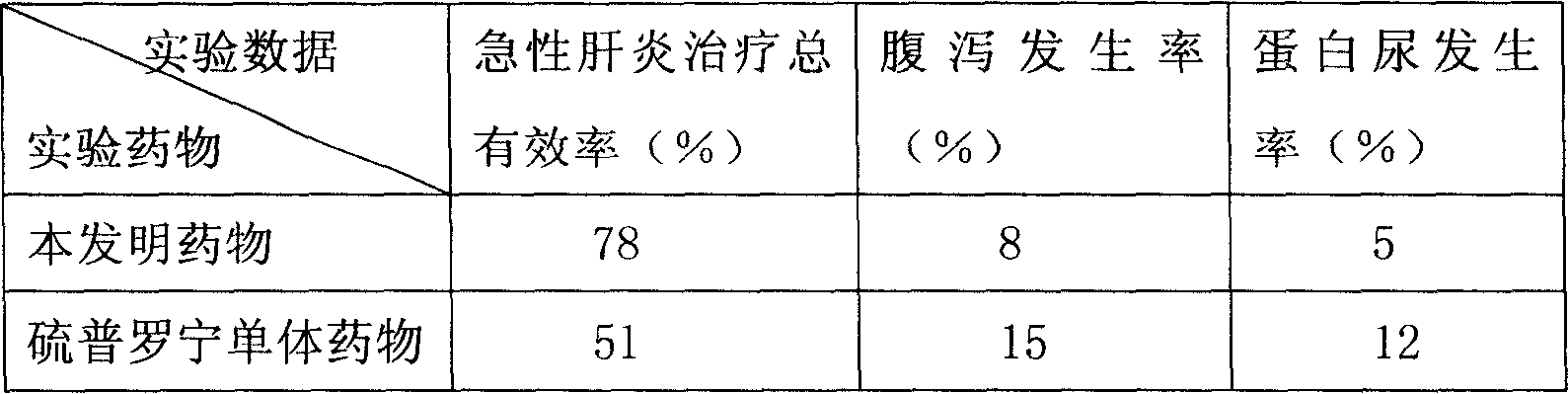
[0034] The present embodiment adopts the mouse of acute hepatitis disease model as experimental animal, carries out comparative experiment with tiopronin combination medicine provided by the present invention and tiopronin monomer medicine respectively, compares grouping, every group 100, body weight 18-20g . In this embodiment and the following examples, the usage and dosage of the tiopronin monomer drug refer to the usage and dosage of the tiopronin combination drug used in the comparative experiment.
[0035] The raw materials that present embodiment adopts and the parts by weight of each raw material are as follows:
[0036] Tiopronin 120;
[0037] Cystine 8;
[0038] Liposome 160;
[0039] Vitamin C 240;
[0040] Monosodium Glutamate 500.
[0041] The preparation method is as follows:
[0042] 1) Get the above-mentioned liposome solution containing 160g liposome.
[0043] 2) Add 120g of tiopronin and 8g of cystine into the liposome solution, stir it ultrasonically ...
Embodiment 2
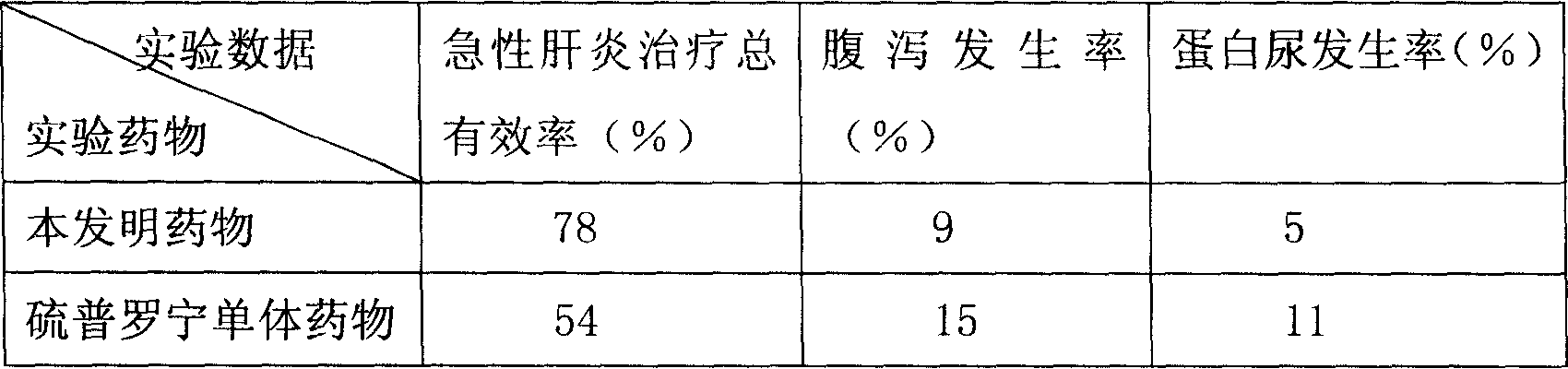
[0053] The present embodiment adopts the mouse of acute hepatitis disease model as experimental animal, carries out comparative experiment with tiopronin combination medicine provided by the present invention and tiopronin monomer medicine respectively, compares grouping, every group 100, body weight 18-20g .
[0054] The raw materials that present embodiment adopts and the parts by weight of each raw material are as follows:
[0055] Tiopronin 260;
[0056] Cystine 4;
[0057] Liposome 300;
[0058] Vitamin C 120;
[0059] Monosodium Glutamate 800.
[0060] The preparation method is as follows:
[0061] 1) Get the above-mentioned liposome solution containing 300g liposome.
[0062] 2) Add 260g of tiopronin and 4g of cystine into the liposome solution, stir it ultrasonically for 10 minutes, and dissolve it completely.
[0063] 3) Add 120g vitamin C and 800g sodium glutamate to the solution obtained in step 2), dissolve and adjust the pH value between 2.5-7.0.
[0064] ...
Embodiment 3
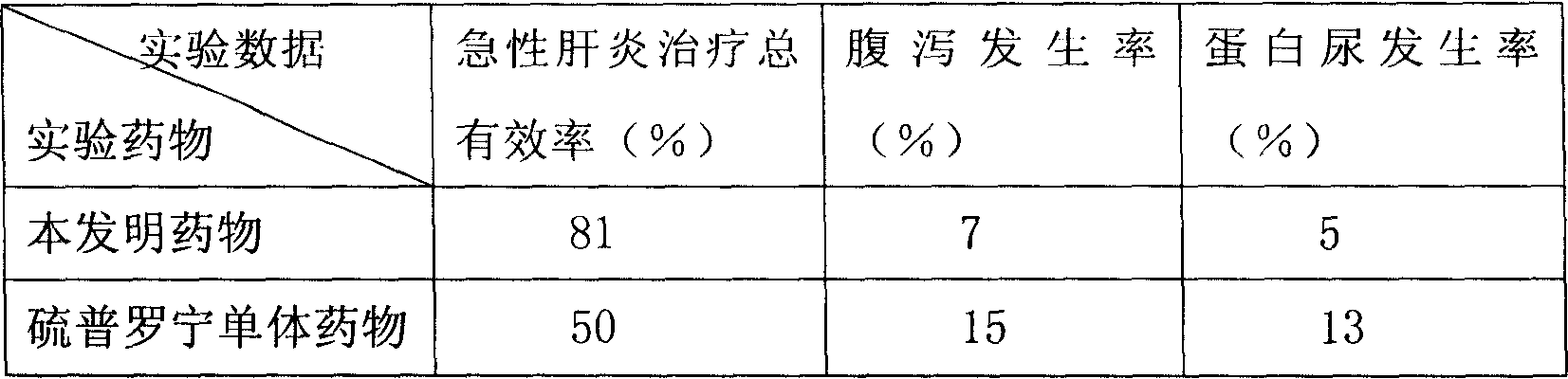
[0072]In this example, the mice of the acute hepatitis disease model were used as experimental animals, and the tiopronin combination drug provided by the present invention and the tiopronin single drug were used for comparative experiments, and the comparison was divided into groups, each group of 100 mice, weighing 18-20g .
[0073] The raw materials used in this embodiment and the parts by weight of each raw material are as follows:
[0074] Tiopronin 120;
[0075] Cystine 4;
[0076] Liposome 300;
[0077] Vitamin C 240;
[0078] Sodium Glutamate 500.
[0079] The preparation method is as follows:
[0080] 1) Take the above-mentioned liposome solution containing 300 g of liposomes.
[0081] 2) 120 g of tiopronin and 4 g of cystine were added to the liposome solution, and ultrasonically stirred for 10 minutes to completely dissolve.
[0082] 3) Add 240 g of vitamin C and 500 g of sodium glutamate to the solution obtained in step 2), dissolve, and adjust the pH value ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com