Preparation and application of silymarin lipid nanoparticles
A technology of lipid nanoparticles and silymarin, applied in the field of biomedicine, can solve the problems of unclear pathogenesis, achieve good brain targeting, good biodegradability, and enhance the effect of drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The construction of embodiment one silymarin lipid nanoparticles
[0038] 1.1 Synthesis of silymarin lipid nanoparticles
[0039] Take 270mg of stearic acid, 90mg of medium-chain triglyceride (MCT) and 40mg of silymarin (SM), add it to 2mL of ethanol, and after heating and melting, inject it into 18mL at a constant rate under a disposable liquid containing 0.1% (mass percentage) Tween 80°C pure water, 60°C magnetic stirring for 5min, stirring at room temperature and cooling to room temperature, milky yellow, the finished product, 4°C low-temperature storage for later use, ultrasonic mixing before each use, the best ready-to-use (the finished product is unstable, put Reconstitution is required after two days).
[0040] 1.2 Size measurement of silymarin lipid nanoparticles
[0041] Take the silymarin nanoparticles synthesized above (SM SLN ) 0.5mL, dilute 10 times with deionized water, and then measure its particle size with a Zetasizer particle size measuring instrume...
Embodiment 2
[0044] Example 2 Intervention and Pharmacodynamics of Silymarin and Its Nanoparticles on the Inflammatory Response of Autism
[0045] Grouping of experimental animals: control group (C57BL / 6NJ), model group (BTBR), three administration groups: silymarin (100mg / kg) group, silymarin lipid nanoparticle (20mg / kg) group, dexamethasone (0.125mg / kg, positive drug) group, with 8 mice in each group. The method of oral administration was adopted, and the administration was continued for 30 days, once a day; the control group (con) and the model group (BTBR) were given normal saline in the same proportion of body weight.
[0046] Evaluation indicators:
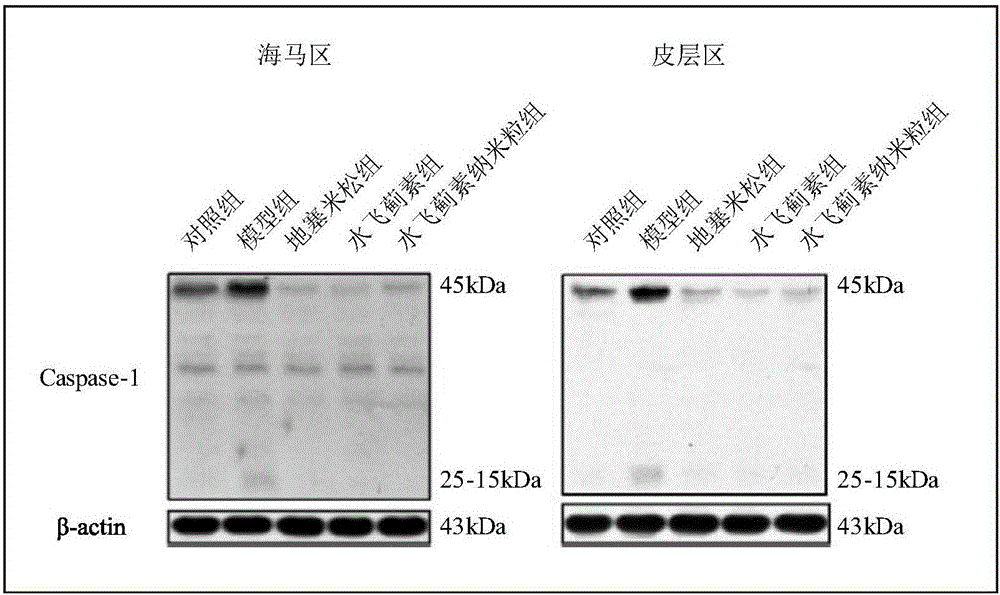
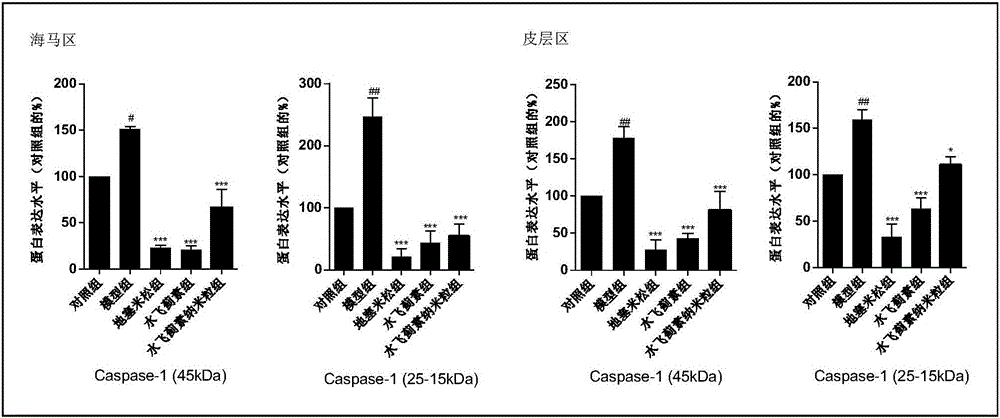
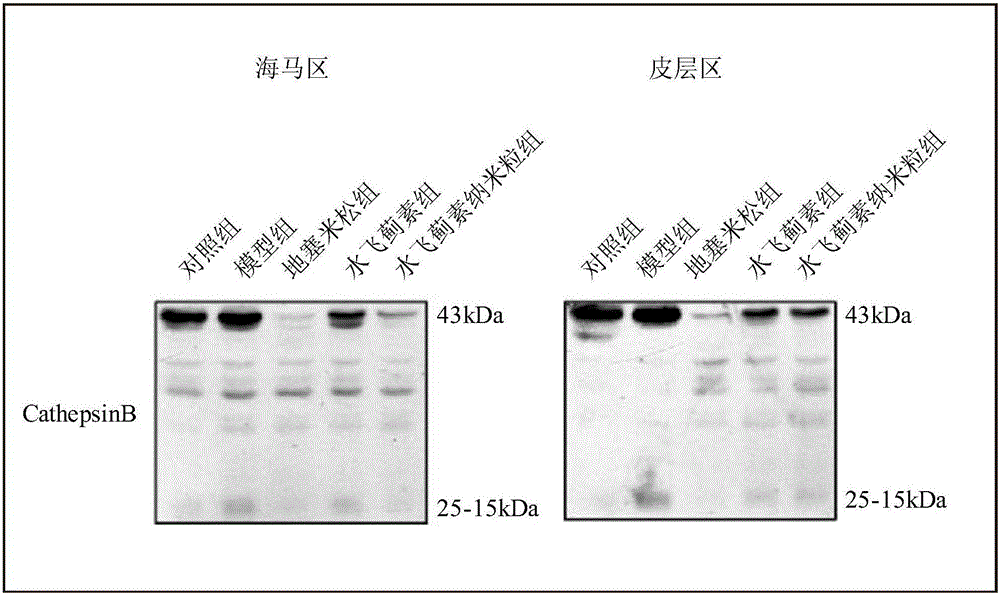
[0047] A. Immunoblot detection: After 30 days of administration, the experimental mice in each group were decapitated and the brains were collected, and the brain tissue samples were collected and homogenized with lysate to lyse the tissue to extract proteins. Western-blotting method was used to examine the inflammation-related protein...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com