A kind of dihydronicotinate derivative and its application
A technology of dihydronicotinic acid esters and derivatives, which is applied in the direction of drug combinations, medical preparations containing active ingredients, cardiovascular system diseases, etc., and can solve the problems of inability to improve the distribution of drugs in the central nervous system and lack of stability. Achieve good brain targeting effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
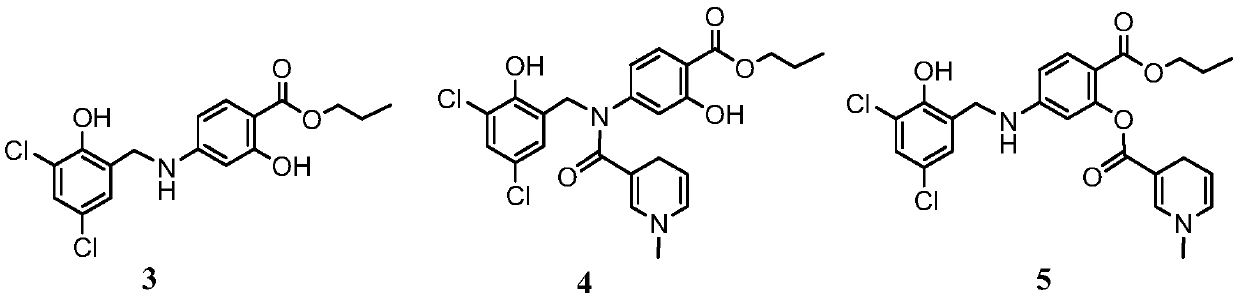
[0020] Example 1 Synthesis of dihydronicotinate derivatives (compound 1) of 4-N-(2-hydroxyl-3,5-dichlorobenzyl)aminosalicylic acid
[0021] The synthetic route of target compound is as follows:
[0022]
[0023] 1) Synthesis of 4-[N-(3,5-dichloro-2-hydroxybenzyl)-N-tert-butoxycarbonyl]amino-2-hydroxybenzoic acid (Boc-ZL006)
[0024] Add 5-(3,5-dichloro-2-hydroxybenzyl)amino-2-hydroxybenzoic acid (1.0g, 3.0mmol), 4-dimethylaminopyridine (0.37g, 3.0mmol) into a 50mL eggplant-shaped bottle , di-tert-butyl dicarbonate (0.65g, 3.0mmol) and dichloromethane (3.0mL), stirred at room temperature for 6h. After the reaction, add water (20mL), extract with ethyl acetate (20mL) 3 times, dry over anhydrous sodium sulfate, and concentrate under reduced pressure to obtain white 5-[N-(3,5-dichloro-2-hydroxybenzyl )-N-tert-butoxycarbonyl]amino-2-hydroxybenzoic acid (1.2 g, 91% yield).
[0025] 1H NMR (300MHz, DMSO-d6) δ7.83(s, 1H), 7.77(d, J=8.43Hz, 1H), 7.69(s, 1H), 6.93(s, 1H), 6.84(d, ...
Embodiment 2
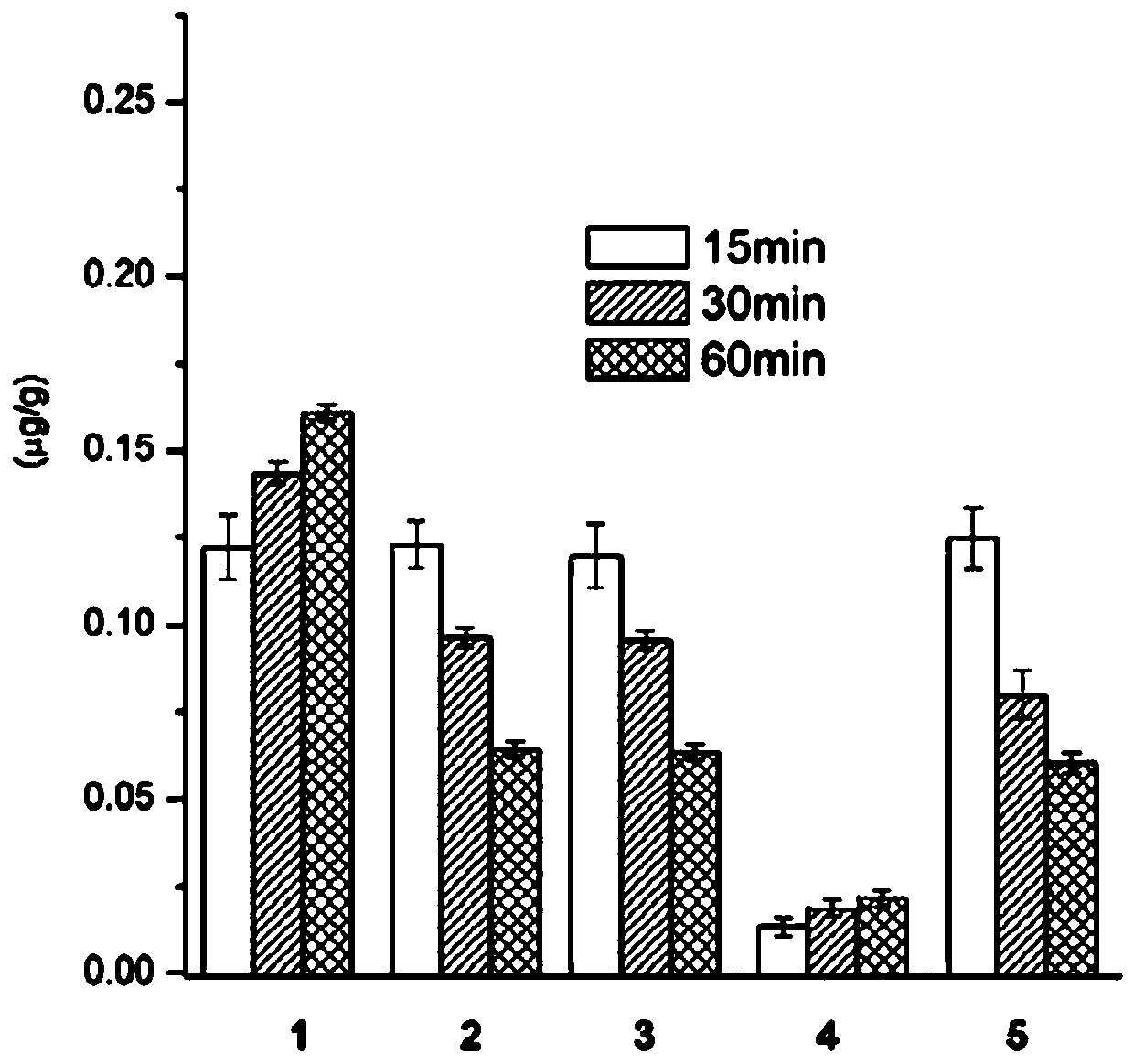
[0030] Concentration determination of ZL006 in embodiment 2 brain tissue
[0031] 1. Preparation of sample solution
[0032] Weigh 20 mg of ZL006 dihydronicotinate (compound 1) sample, dissolve it in a very small amount of DMSO (less than 1% of the total volume), add Tween 80 (less than 5% of the total volume), and add Dilute normal saline to 20mL, and prepare to a concentration of 1mg·mL -1solution.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com