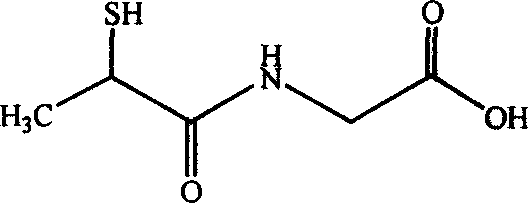
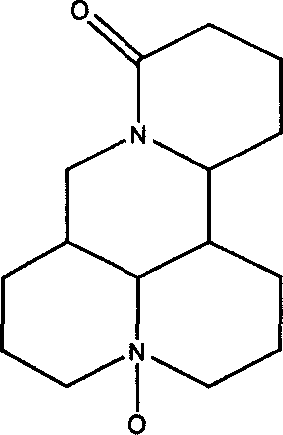
Anti-hepatitis medical combination
A composition and drug technology, applied in the field of medicine, to achieve the effect of improving liver cell function, scavenging free radicals, and remarkable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0170] The preparation of embodiment 1 rhodiola rosea polysaccharide
[0171] Take rhodiola rosea medicinal material, crush it, pass through a 40-mesh sieve, add 10 times the amount of water, soak for half an hour, put it into an ultrasonic extraction tank, extract by ultrasonic oscillation for 40 minutes, and the oscillation frequency is 60kHz, extract 3 times, combine the extracts, and filter , and the filtrate was concentrated under reduced pressure into an extract. Add 30 times the amount of water at 60°C to the extract, stir, stand still, pour out the supernatant, concentrate under reduced pressure until 1ml of the medicinal solution contains 1g of medicinal material, add ethanol until the alcohol content is 80%, and let stand for 24 hours. Filter, collect the precipitate, add 30 times the amount of water to dissolve the precipitate, add 1% tannic acid dropwise under stirring, heat, boil, and centrifuge to remove the precipitate, continue to add tannic acid until the solu...
Embodiment 2
[0183] The preparation of embodiment 2HG, HL, HK, HS composition powder injection
[0184] 1. Prescription:
[0185] Prescription 1:
[0186] Rhodiola polysaccharide 20g (equivalent to 1kg Rhodiola)
[0187] Monoammonium Glycyrrhizinate 40g
[0188] Polysorbate 80 35g
[0189] Mannitol 300g
[0190] Add sterile water for injection to 5000ml
[0191] A total of 1000 sticks were prepared
[0192] Prescription 2:
[0193]Rhodiola polysaccharide 20g (equivalent to 1kg Rhodiola)
[0194] Tiopronin 100g
[0195] Polysorbate 80 35g
[0196] Mannitol 300g
[0197] Add sterile water for injection to 5000ml
[0198] A total of 1000 sticks were prepared
[0199] Prescription 3:
[0200] Rhodiola polysaccharide 20g (equivalent to 1kg Rhodiola)
[0201] Matrine 400g
[0202] Polysorbate 80 35g
[0203] Mannitol 300g
[0204] Add sterile water for injection to 5000ml
[0205] A total of 1000 sticks were prepared
[0206] Prescription 4:
[0207] Rhodiola polysaccharide 2...
Embodiment 3
[0224] The preparation of embodiment 3HG, HL, HK, HS composition aqueous injection
[0225] 1. Prescription:
[0226] Prescription 1:
[0227] Rhodiola polysaccharide 20g (equivalent to 1kg Rhodiola)
[0228] Diammonium Glycyrrhizinate 150g
[0229] Add water for injection to 5000ml
[0230] A total of 1000 sticks were prepared
[0231] Prescription 2:
[0232] Rhodiola polysaccharide 20g (equivalent to 1kg Rhodiola)
[0233] Tiopronin 100g
[0234] Add water for injection to 5000ml
[0235] A total of 1000 sticks were prepared
[0236] Prescription 3:
[0237] Rhodiola polysaccharide 20g (equivalent to 1kg Rhodiola)
[0238] Matrine 400g
[0239] Add water for injection to 5000ml
[0240] A total of 1000 sticks were prepared
[0241] Prescription 4:
[0242] Rhodiola polysaccharide 20g (equivalent to 1kg Rhodiola)
[0243] Silybin Meglumine 100g
[0244] Add water for injection to 5000ml
[0245] A total of 1000 sticks were prepared
[0246] 2. Specific step...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com