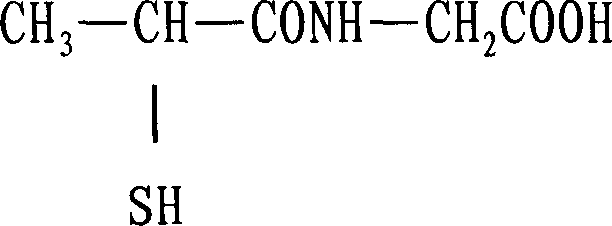
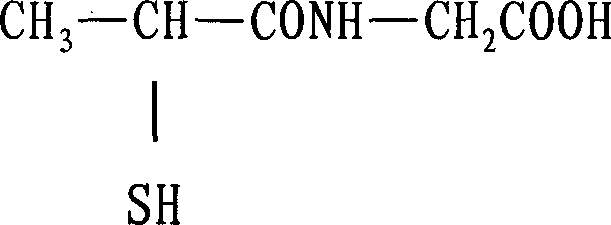
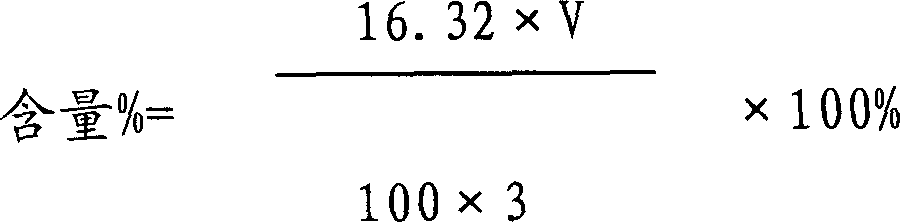Tiopronin freeze-dried powder injection
A technology of tiopronin and needle injection, which is applied in the field of hepatoprotective drug tiopronin and its freeze-dried powder injection and its preparation, can solve the problems of short validity period, poor stability of tiopronin, unsuitability, etc., and achieve convenient use , excellent curative effect and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0220] Embodiment 1: preparation tiopronin freeze-dried powder injection
[0221] Tiopronin 100g
[0222] Dextran 40 50g
[0223] Water for injection 1000ml
[0224] A total of 1000 freeze-dried products were made
[0225] Preparation Process:
[0226] Preparation of liquid medicine:
[0227] Weigh the prescribed amount of raw materials for injection (the content of tiopronin must not be less than 98.5%, and the content of heavy metals must not exceed 15 parts per million), add sterile pyrogen-free distilled water to dissolve, and the concentration is 0.2g / ml. Then add the prescribed amount of 20% dextran 40 solution that has been sterilized and depyrogenated in advance and mix well. Add water to 1000ml, so that the content of tiopronin is 0.1g / ml, and the content of dextran 40 is 0.05 / ml;
[0228] Depyrogenation:
[0229] Add activated carbon for injection into the above liquid medicine (the amount of activated carbon is 0.3-0.5% of the weight of the tiopronin raw mate...
Embodiment 2
[0238] Embodiment 2: preparation tiopronin freeze-dried powder injection
[0239] Tiopronin 100g
[0240] Lactose 50g
[0241] Mannitol 50g
[0242] Water for injection 1000ml
[0243] A total of 1000 freeze-dried products were made
[0244] The preparation method is the same as in Example 1 except that the auxiliary materials added are different.
Embodiment 3
[0245] Embodiment 3: preparation tiopronin freeze-dried powder injection
[0246] Tiopronin 100g
[0247] Lactose 50g
[0248] Water for injection 1000ml
[0249] A total of 1000 freeze-dried products were made
[0250] The preparation method is the same as in Example 1 except that the auxiliary materials added are different.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com