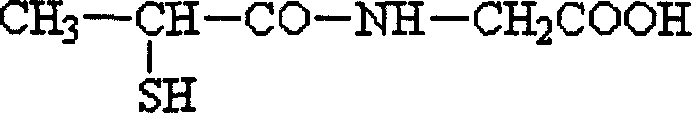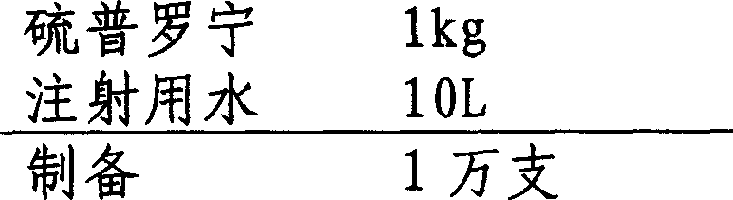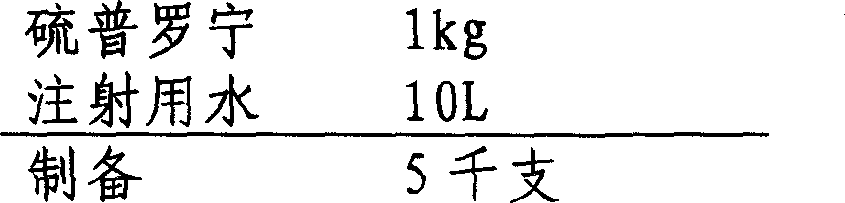Freeze dry tiopronin preparation without adjuvant for intravenous injection and its preparation process
A technique for tiopronin and freeze-dried preparations, which is applied in the field of freeze-dried preparations for intravenous injection of tiopronin and the preparation process, which can solve the problems of physical harm to patients, increased production costs of preparations, complexity of prescriptions and difficulty in drug quality control, etc. , to achieve the effects of guaranteed safety, easy control of preparation quality, and reduced production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] 1. Preparation prescription:
[0019]
[0020] Second, the preparation process:
[0021] (1) Preparation of liquid medicine
[0022] 1. Take an appropriate amount of freshly prepared water for injection (about 80% of the prescription), lower the temperature to below 30°C, add the prescribed amount of tiopronin, and stir to dissolve;
[0023] 2. Add activated carbon (0.2% w / v), stir and adsorb for 10 minutes, and decarbonize by filtration with a 0.45 μm microporous membrane.
[0024] 3. Add water for injection below 30°C to the prescribed amount and stir well;
[0025] 4. Detect the drug content of the filtrate, and use a 0.22μm microporous membrane to filter and sterilize;
[0026] 5. After the clarity is qualified, it is divided into small glass bottles (1ml / bottle, the dose is 100mg).
[0027] (2) freeze-drying process
[0028] 1. Put the sample into the freeze-drying box, lower it to -40°C (1°C to 2°C per minute), and keep it warm for 2 hours; start vacuumi...
Embodiment 2
[0032] 1. Preparation prescription:
[0033]
[0034] Second, the preparation process:
[0035] (1) Preparation of liquid medicine
[0036] 1. Take an appropriate amount of freshly prepared water for injection (about 80% of the prescription), lower the temperature to below 30°C, add the prescribed amount of tiopronin, and stir to dissolve;
[0037] 2. Add activated carbon (0.2% w / v), stir and adsorb for 10 minutes, and decarbonize by filtration with a 0.45 μm microporous membrane.
[0038] 3. Add water for injection below 30°C to the prescribed amount and stir well;
[0039] 4. Detect the drug content of the filtrate, and use a 0.22μm microporous membrane to filter and sterilize;
[0040] 5. After the clarity is qualified, it is divided into small glass bottles (2ml / bottle, the dose is 200mg).
[0041] (2) freeze-drying process
[0042] 1. Put the sample into the freeze-drying box, lower it to -60°C (1°C to 2°C per minute), and keep it warm for 2 hours; start vacuumi...
Embodiment 3
[0045] 1. Preparation prescription:
[0046]
[0047] Second, the preparation process:
[0048] (1) Preparation of liquid medicine
[0049] 1. Take an appropriate amount of freshly prepared water for injection (about 80% of the prescription), lower the temperature to below 30°C, add the prescribed amount of tiopronin, and stir to dissolve;
[0050] 2. Add activated carbon (0.2% w / v), stir and adsorb for 10 minutes, and decarbonize by filtration with a 0.45 μm microporous membrane.
[0051] 3. Add water for injection below 30°C to the prescribed amount and stir well;
[0052] 4. Detect the drug content of the filtrate, and use a 0.22μm microporous membrane to filter and sterilize;
[0053] 5. After the clarity is qualified, it is divided into small glass bottles (0.5ml / bottle, the dose is 100mg).
[0054] (2) freeze-drying process
[0055] 1. Put the sample into the freeze-drying box, lower it to -40°C (1°C to 2°C per minute), and keep it warm for 2 hours; start vacuu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com