Thipronin enteric-coated delayed-release agent
A tiopronin enteric and slow-release preparation technology, which is applied in antidote, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problems of large fluctuations in drug concentration and reduce direct stimulation Sexuality, good for long-term treatment, avoid nausea effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
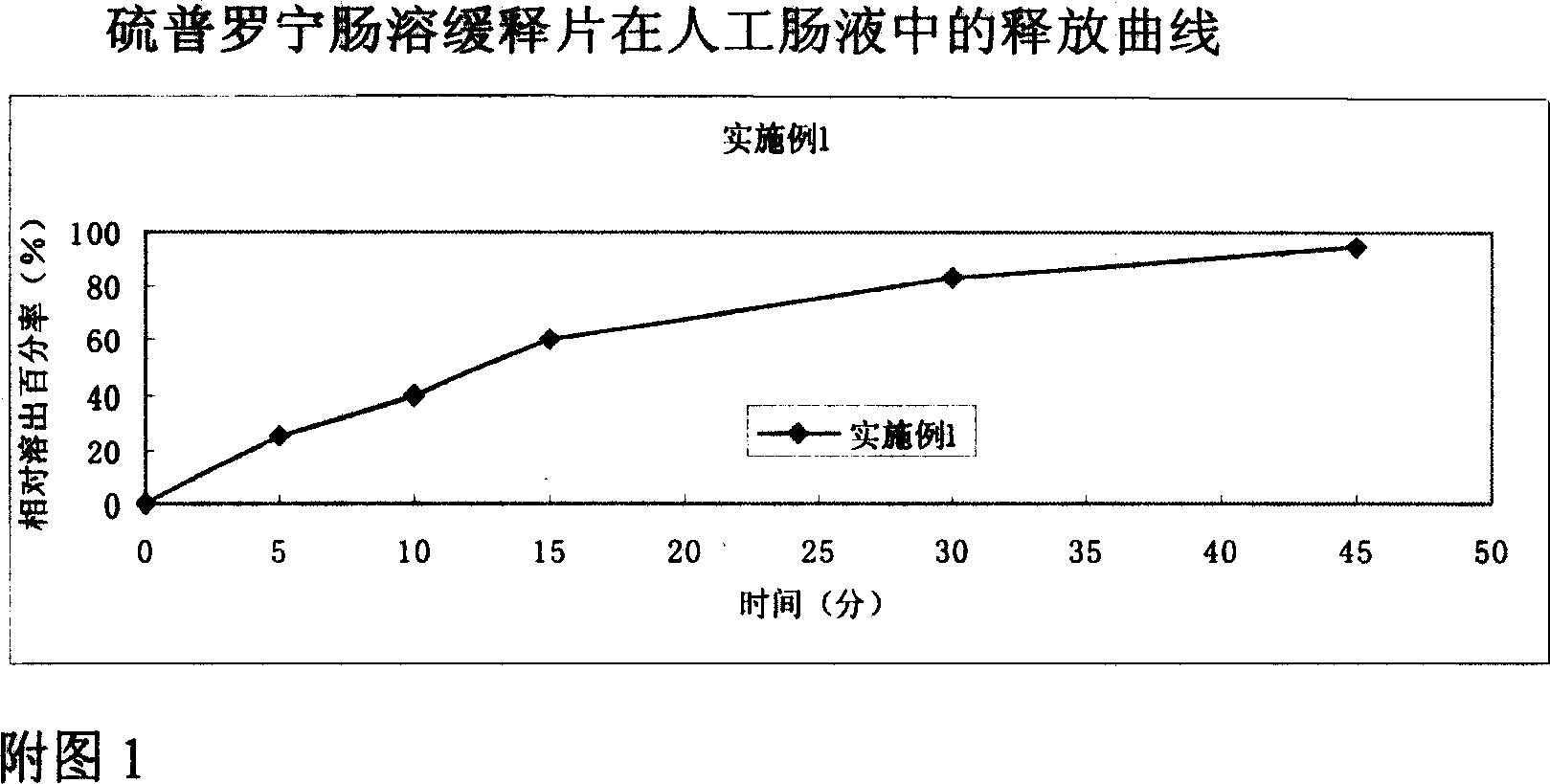
Embodiment 1
[0022] Preparation of Tiopronin Tablets:
[0023] prescription:
[0024] Names of raw and auxiliary materials (1000 tablets)
[0025] Tiopronin 300.0g
[0026] Microcrystalline Cellulose 50.0g
[0027] Pregelatinized starch 50.0g
[0028] 15% povidone K30 ethanol solution 150ml
[0029] Magnesium Stearate 6.0g
[0030] Preparation Process:
[0031] Weigh the prescribed amount of tiopronin, microcrystalline cellulose, and pregelatinized starch fully and evenly, add an appropriate amount of binder to make a soft material, granulate, and dry at 50°C; for whole granules, add the prescribed amount of stearic acid Magnesium is thoroughly mixed and pressed into tablets.
[0032] Dissolution Determination:
[0033] According to the release assay method (Chinese Pharmacopoeia version two appendix XD second method (two) method in 2005), the first method device of the dissolution assay method is adopted, and phosphate buffer (pH6.8) 500ml is used as solvent, and the rotating spee...
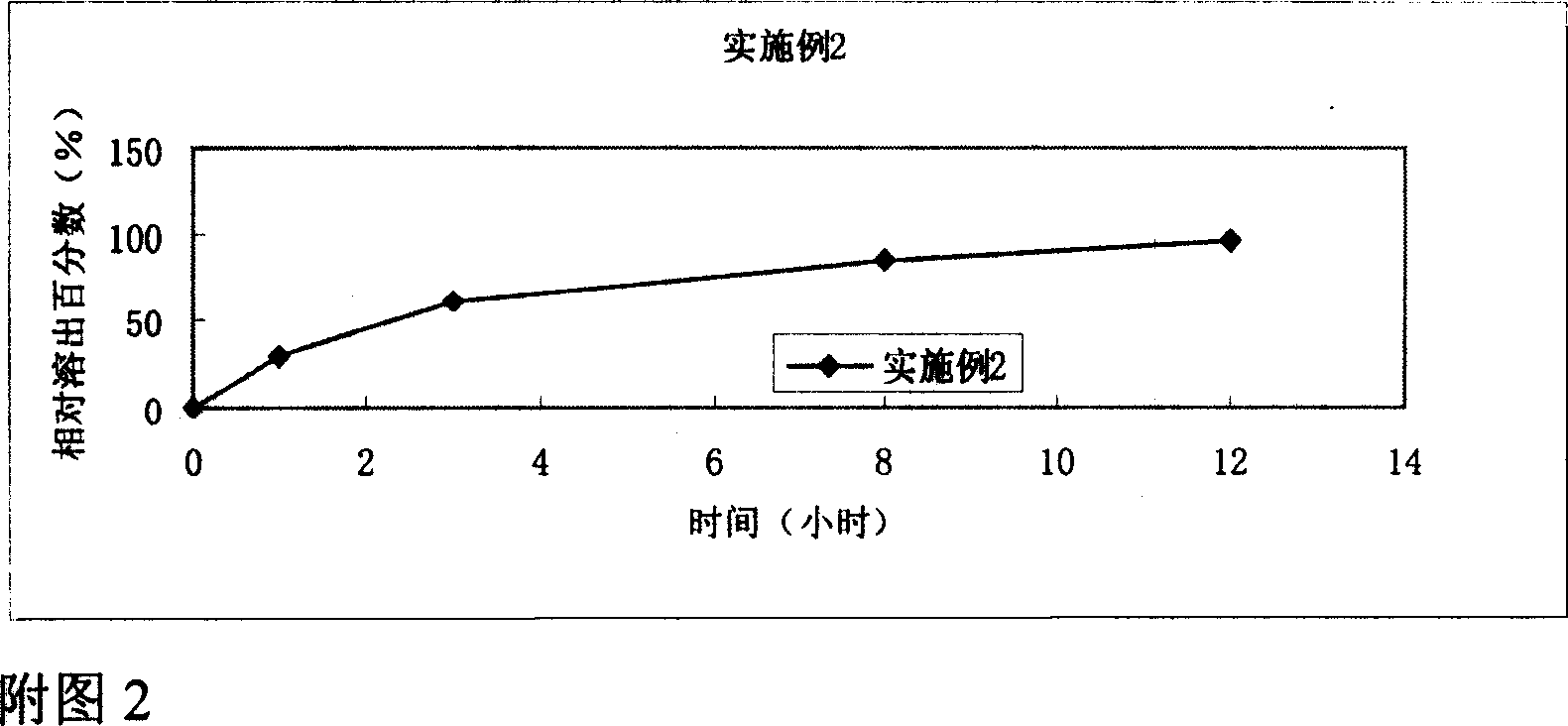
Embodiment 2
[0035] Preparation of Tiopronin Enteric-coated Sustained-release Tablets (1000 Tablets Quantity)
[0036] Sustained release layer prescription:
[0037] Tiopronin 300.0g
[0038] Hypromellose K4MCR 100.0g
[0039] Hypromellose K100MCR 75.0g
[0040] Microcrystalline Cellulose 50.0g
[0041] Pregelatinized starch 50.0g
[0042] 15% povidone K30 ethanol solution 150ml
[0043] Magnesium Stearate 6.0g
[0044] Enteric coating layer prescription:
[0045] Ethyl cellulose 60.0g
[0046] Macrogol 4000 28.0g
[0047] 95% ethanol 2000ml
[0048] Preparation Process:
[0049] Weigh the prescribed amount of tiopronin, microcrystalline cellulose, pregelatinized starch, hypromellose K4MCR, hypromellose K100MCR fully uniform, add an appropriate amount of binder to make a soft material, granulate, 50 ℃ Blow drying; whole grain, add prescription amount of magnesium stearate, mix thoroughly, and compress into tablets. Spray coating solution and enteric coating.
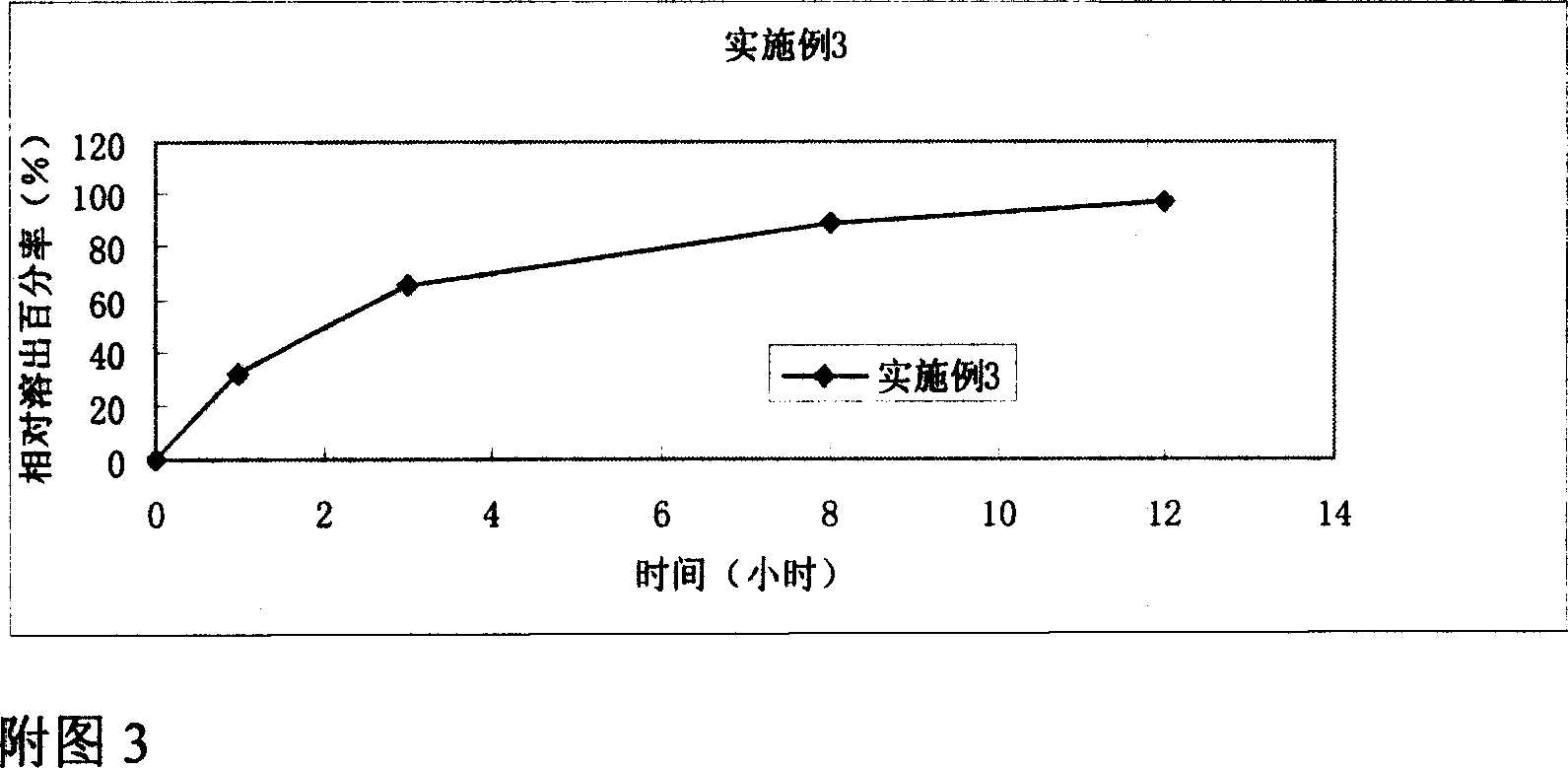
Embodiment 2~11
[0051] According to the release assay method (Chinese Pharmacopoeia version two appendix XD second method (two) method in 2005), the dissolution assay method first method device is adopted, with 0.1mol / L hydrochloric acid solution 500ml as solvent, and the rotating speed is 100 revolutions per minute , operate according to law. After 2 hours, no cracks or disintegration should be found on the test pieces. Precisely measure 10ml, filter, take the filtrate to measure, the released amount must not exceed 10% of the marked amount; take the above test piece and add 500ml of phosphate buffer (pH6.8) immediately, keep the speed constant, continue to operate according to the law, Accurately measure 10ml of the solution for a certain period of time, and add 10ml of phosphate buffer (pH6.8) at the same temperature to the operating container in time, and take the subsequent filtrate for determination.
[0052] The results showed that the tiopronin sustained-release tablets prepared with...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com