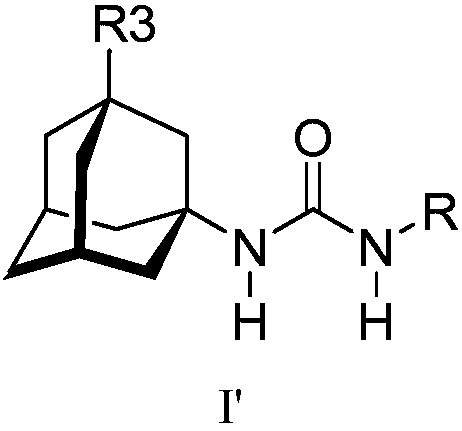
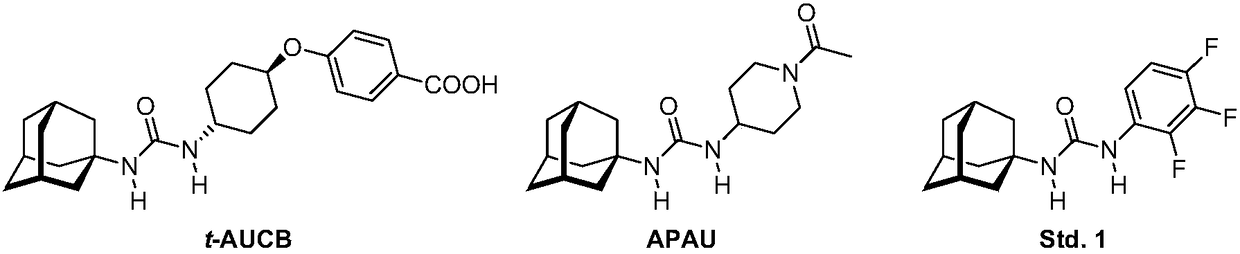
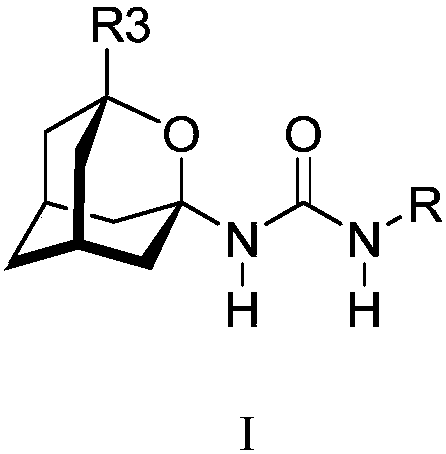
Analogs of adamantylureas as soluble epoxide hydrolase inhibitors
An alkyl, compound technology, applied in the field of analogs of adamantyl urea as a soluble epoxidase inhibitor, can solve the problems of low water solubility, lack of selectivity, chemical and metabolic instability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1a
[0084] Example 1a: Preparation of 1-(2-oxaadamantan-1-yl)-3-(2,3,4-trifluorophenyl)urea, I a
[0085]
[0086] In a round bottom flask equipped with a stir bar, 1.2 eq. of (2-oxaadamantan-1-yl)amine hydrochloride was added to anh. dichloromethane (DCM) (-110 mM) under nitrogen atmosphere. To this suspension was added 1.0 eq. of 2,3,4-trifluorophenylisocyanate followed by 7 eq. of triethylamine (TEA). The reaction mixture was stirred overnight at room temperature. The solvent was then removed under vacuum and the resulting crude product (SiO 2 , hexane / ethyl acetate mixture), and evaporation of the appropriate fractions in vacuo afforded urea I as a white solid a(163 mg, 94% yield), mp 196-198°C. IR (ATR): 3300-2800 (3293, 3232, 3127, 2933, 2857), 1702, 1640, 1621, 1563, 1509, 1489, 1471, 1446, 1373, 1349, 1340, 1317, 1294, 1257, 1239, 1227, 1200, 1165, 1117, 1099, 1080, 1020, 996, 976, 963, 932, 912, 884, 840, 805, 788, 757, 683, 653cm -1 . MS(DIP), m / e(%): 179(11), ...
Embodiment 1b
[0087] Example 1b: Preparation of 1-(3-methyl-2-oxaadamantan-1-yl)-3-(2,3,4-trifluorophenyl)urea, I b
[0088]
[0089] Using (3-methyl-2-oxaadamantan-1-yl)amine in a procedure analogous to one of the examples 1a, the title compound was obtained in 93% yield. Mp 195-197°C. IR (ATR): 3300-2800 (3270, 3227, 3128, 2976, 2927, 2856), 1701, 1641, 1622, 1564, 1509, 1492, 1471, 1373, 1341, 1322, 1301, 1286, 1256, 1228, 1213, 1200, 1171, 1136, 1106, 1090, 1072, 1034, 1006, 991, 972, 959, 921, 899, 885, 804, 788, 755, 682, 670, 652cm -1 . MS(DIP), m / e(%): 172(13), 150(14), 149(100), 148(80), 147(25), 109(10), 108(14), 107(11 ), 95(10), 93(25). Analytical Computing C 17 h 19 f 3 N 2 o 2 0.05H 2 O: C 59.84, H 5.64, F 16.70, N 8.21. Found: C 59.91, H 5.90, F 16.52, N 8.22.
Embodiment Ic
[0090] Example Ic: Preparation of 1-(3-ethyl-2-oxaadamantan-1-yl)-3-(2,3,4-trifluorophenyl)urea, I c
[0091]
[0092] Using (3-ethyl-2-oxaadamantan-1-yl)amine in a procedure analogous to one of the examples 1a, the title compound was obtained in 96% yield. Mp 165-166°C. IR (ATR): 3300-2800 (3288, 3238, 3128, 2970, 2927, 2850), 1702, 1641, 1622, 1563, 1509, 1471, 1371, 1341, 1322, 1301, 1254, 1227, 1209, 1172, 1091, 1010, 996, 965, 939, 921, 896, 803, 788, 755, 669, 653cm -1 . MS (DIP), m / e (%): 354 (M ·+ , 5), 148(14), 146(100), 94(10), 93(10). Analytical Computing C 18 h 21 f 3 N 2 o 2 • 0.01 EtOAc: C 60.99, H 5.98, F 16.04, N 7.89. Found: C 60.97, H 6.06, F 16.23, N 7.84.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com