Sulfonamide derivatives and methods of use thereof for improving the pharmacokinetics of a drug
A pharmacy and drug technology, applied in the field of improving the pharmacokinetics of drugs, can solve problems such as unfavorable pharmacokinetics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
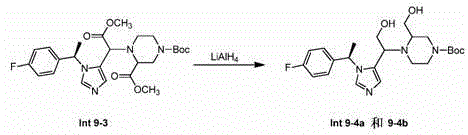
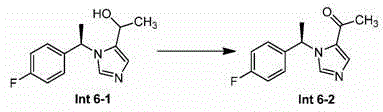
[0318] Preparation of compound 1
[0319]
[0320] Preparation of Step A-Int (Intermediate) 1-1
[0321]
[0322] To a solution of 1-(4-fluoro-phenyl)-ethanone (400 g, 2.9 mol) and 2-methylpropane-2-sulfinic acid amide (350 g, 2.9 mol) in THF (4 L) Add Ti(OiPr) in 4 (824 g, 2.9 mol). The reaction mixture was stirred at 90°C for 48 hours until complete when indicated by TLC (PE:EA=10:1). The mixture was cooled to 0 °C, NaBH was added in portions 4(110 g, 209 mol). The reaction was stirred at 0 °C until TLC (petroleum ether / EtOAc = 1:1) indicated completion of the reaction (~1 hr). The reaction mixture was poured into ice water, and the resulting precipitate was filtered off and washed with ethyl acetate. The filtrate was concentrated in vacuo, then dissolved in water, the aqueous layer was extracted with ethyl acetate (800 mL x 3), and dissolved in anhydrous Na 2 SO 4 Drying in , and concentration in vacuo gave the resulting residue, Intermediate 1-1, which was pu...
Embodiment 2
[0362] Preparation of Compound 28
[0363]
[0364] Preparation of step A intermediate 2-1
[0365]
[0366] Potassium tert-butoxide (2.8 g, 24.84 mmol) was dissolved in DMF (10 mL), and benzyl mercaptan (2.6 g, 20.70 mmol) was added dropwise at 0°C. The mixture was stirred at room temperature for 15 minutes, then cooled to 0 °C, a solution of 5-bromo-2-chloropyridine (4.0 g, 20.70 mmol) in DMF (4 mL) was added dropwise at 0 °C, followed by The mixture was heated at 80°C for 1.5 hours. The mixture was poured into water (100 mL), extracted with ethyl acetate (3 x 100 mL). The combined organic phases were washed with brine (100 mL), water (100 mL), and dried (Na 2 SO 4). Concentration in vacuo gave a residue, which was purified by column chromatography (petroleum ether:EtOAc=10:1) to give Intermediate 2-1 (4.0 g, 69%). MS(ESI): m / z (M+H) + 280.
[0367] Preparation of step B intermediate 2-2
[0368]
[0369] To a solution of Intermediate 2-1 (1.5 g, 5.40 mm...
Embodiment 3
[0380] Preparation of compound 31
[0381]
[0382] Preparation of step A intermediate 3-1
[0383]
[0384] At 0°C, under nitrogen, to 5-bromo-2-cyanopyridine (30.0 g, 0.165 mol) and Ti(O-iPr) under stirring 4 (51.5 g, 0.181 mol) in 900 mL THF was added EtMgBr (330 mL, 0.045 mmol). The reaction mixture was stirred at room temperature for 5 hours. The reaction was quenched with water, extracted with EtOAc, filtered, and the organic layer was dissolved in Na 2 SO 4 Dry in , filter and concentrate in vacuo. The resulting residue was purified by flash column chromatography on silica gel, eluting with petroleum ether:EtAOc=30:1 to obtain the product (7.2 g, 21%). MS-ESI ( m / z ): 213, 215 (M+H) + .
[0385] Preparation of step B intermediate 3-3
[0386]
[0387] To compound Int3-1 (7.1 g, 0.033 mol) in 80 mL mixed solvent (MeCN / H 2 O=1:1) was added 2,5-bis(hydroxymethyl)-1,4-dioxane-2,5-diol (7.81 g, 0.043 mol), KSCN (4.18 g, 0.043 mol) and acetic acid (4 mL)....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com