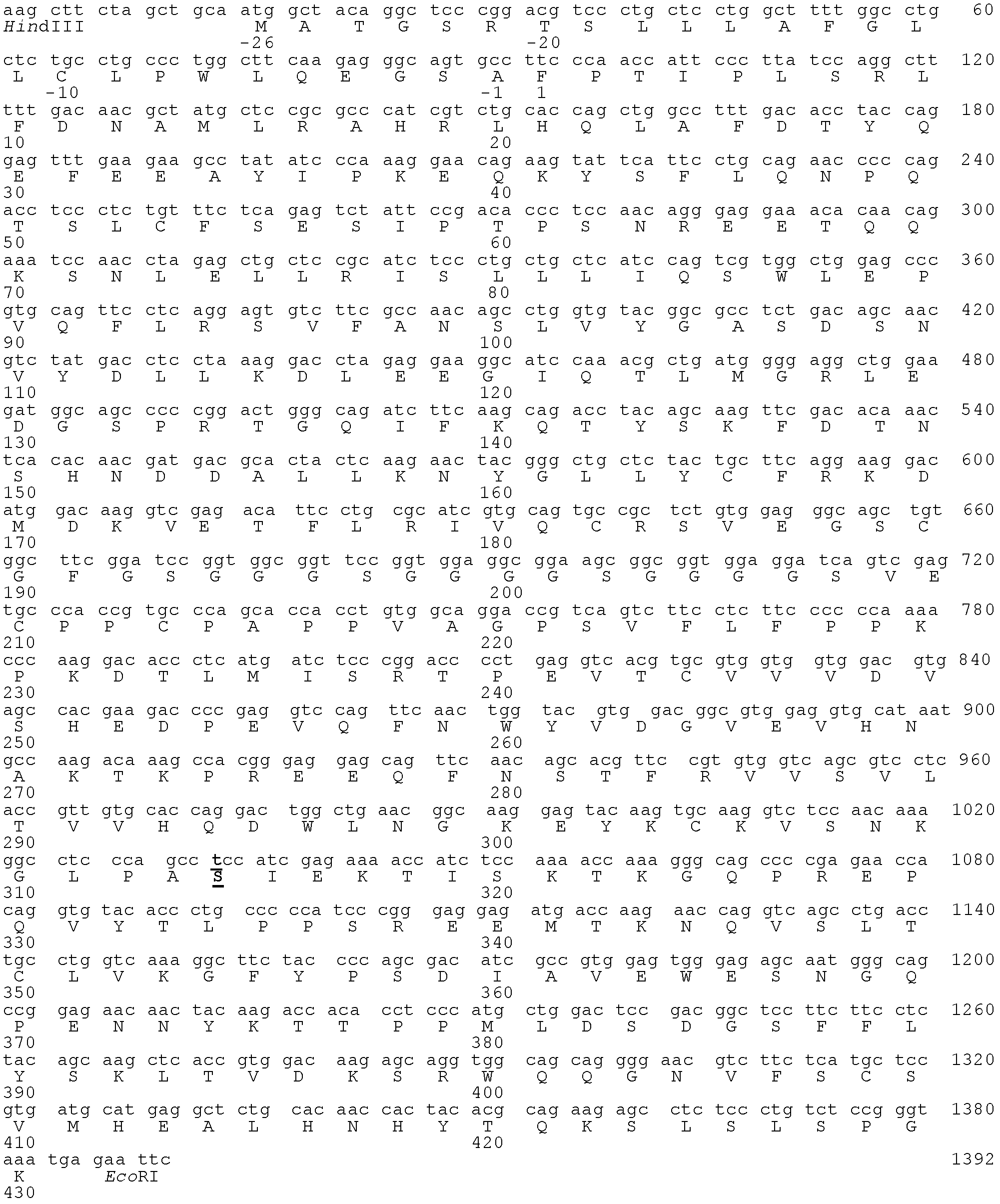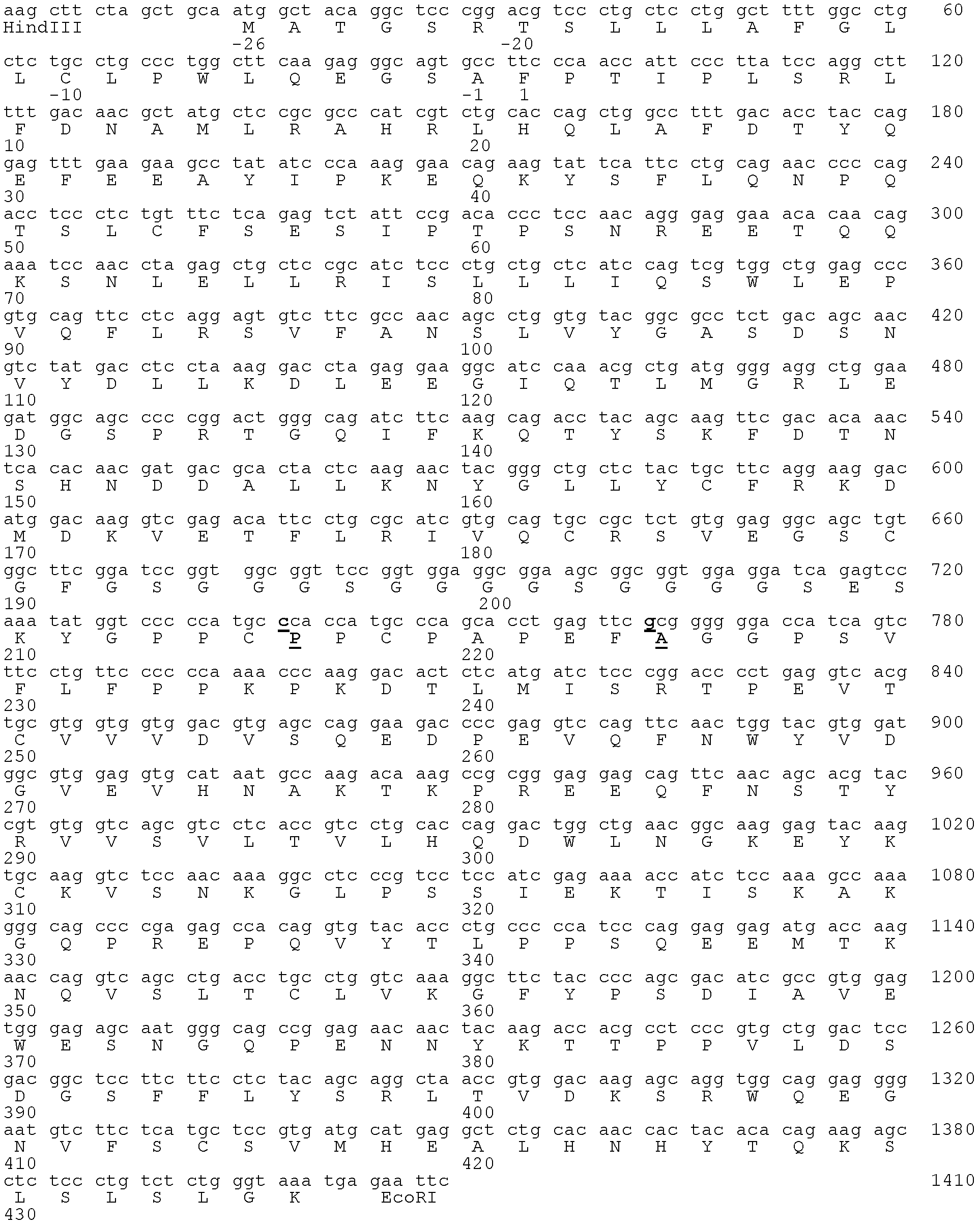Fc fusion protein of long-acting recombinant human growth hormone
A fusion protein and biological activity technology, applied in the fields of molecular biology and medicine, can solve the problems of difficult construction of hGH-L-vFc fusion protein, no half-life GH derivatives, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] Example 1 Construction of encoding hGH-L-vFc γ2 gene encoding the fusion protein
[0082] 1. Preparation of human-growth hormone-containing gene sequence plasmid
[0083] hGH-L-vFc γ2 The fusion protein coding sequence is composed of several DNA fragments. The gene encoding the leader peptide and mature protein containing human GH is prepared by artificial synthesis method according to the human-growth hormone gene sequence contained in NCBI reference number NM_000515.3. The synthesis method is to advance along the double-stranded DNA sequence of the hGH gene from the 5' terminal to the 3' terminal, and prepare four oligonucleotide fragments with alternate upper and lower chains and a length of about 180 bases. The end of each fragment contains about 20 base complementary overlapping sequences with the end of the next complementary chain fragment respectively. Afterwards, the four oligonucleotide fragments were combined into a nucleotide fragment with a length of ab...
Embodiment 2
[0096] Example 2 Construction of encoding hGH-L-vFc γ4 gene encoding the fusion protein
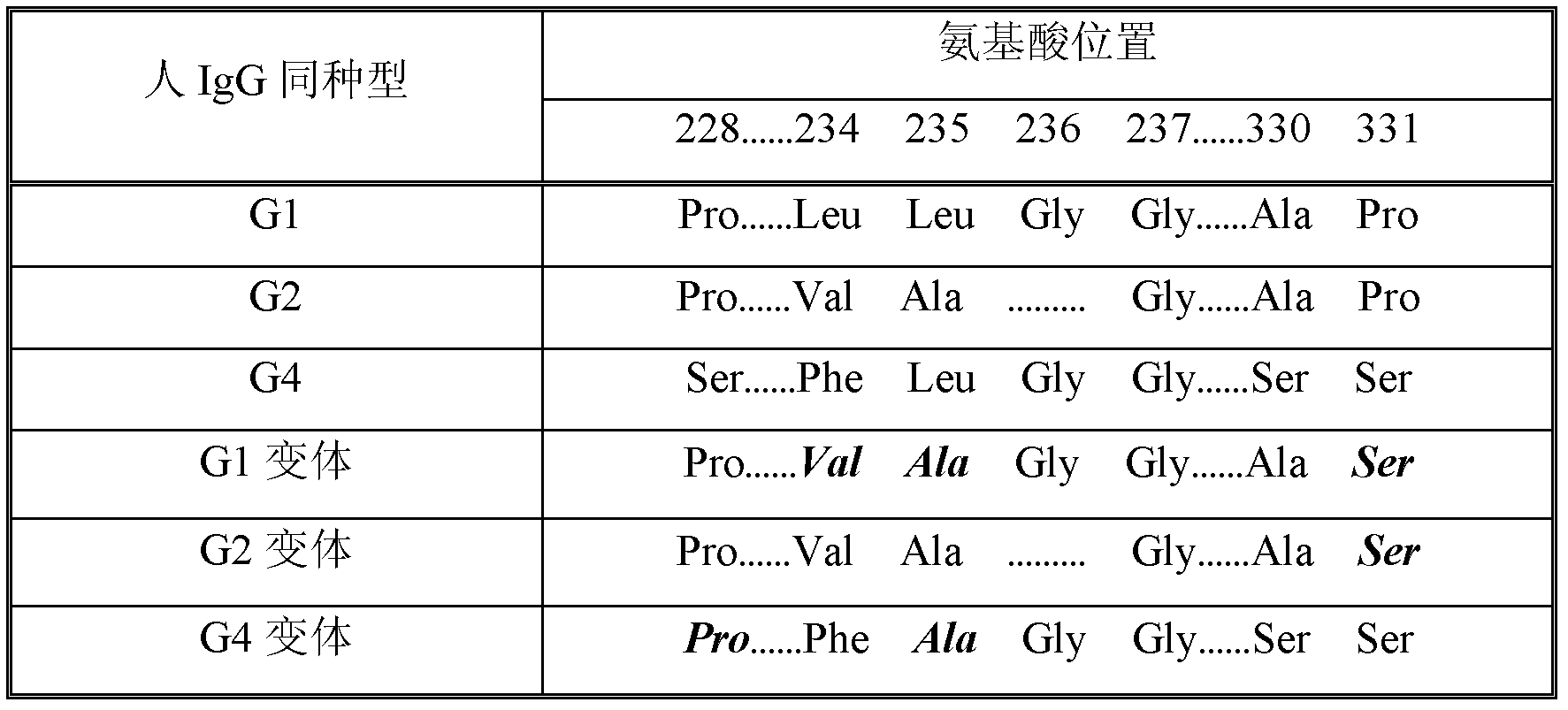
[0097] Due to dissociation of the inter-heavy chain disulfide bonds in the hinge region, a portion of human IgG4 dissociates to form what is considered a half-antibody molecule. This situation does not normally occur in molecules of the other three human IgG isotypes. The literature shows that the 228 site in IgG1 and IgG2 is Pro, while the site is Ser228 in IgG4. Substituting the Ser228 residue of IgG4 with Pro as a single amino acid can keep IgG4 an intact antibody molecule (see Angal et al., Molec. Immunol., 30:105-108, 1993; Owens et al., Immunotechnology, 3:107-116, 1997; US Patent No. 6,204,007). Additionally, the Fc γ4 Carry out Leu235Ala mutation, can make this Fc γ4 The variants have reduced binding to FcyRs. This mutation, together with the aforementioned Ser228Pro mutation, will allow the fusion protein to obtain a more uniform and complete preparation during purification...
Embodiment 3
[0102] Example 3 Construction of encoding hGH-L-vFc γ1 gene encoding the fusion protein
[0103]The hinge region of the human IgGl heavy chain contains 15 amino acid residues including 3 cysteines (GluProLysSerCysAspLysThrHisThrCysProProCysPro). Among these 3 cysteine residues, the 2nd and 3rd are involved in the formation of disulfide bonds between the two heavy chains. The first cysteine residue is involved in disulfide bonding to the IgG light chain. Since there is no light chain in the Fc fusion protein molecule, this cysteine residue may pair with other cysteine residues, resulting in non-specific disulfide bonding. To prevent this non-specific disulfide bonding, the Fc γ1 The hinge region of was shortened to eliminate the first cysteine residue (AspLysThrHisThrCysProProCysPro). Using RNA prepared from human leukocytes and appropriate 5' primers (SEQ ID NO: 13) and 3' primers (SEQ ID NO: 4), Fc-containing γ1 Region-encoding genes. The resulting Fc γ1 DNA ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com