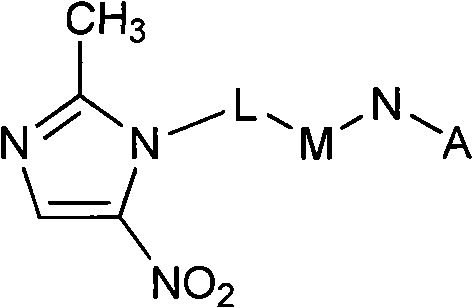
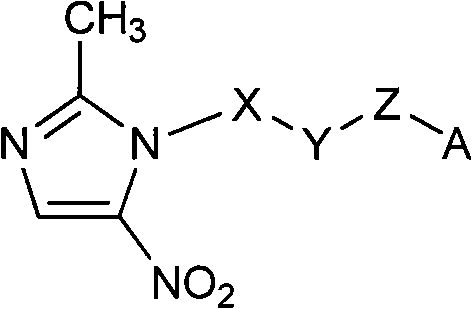
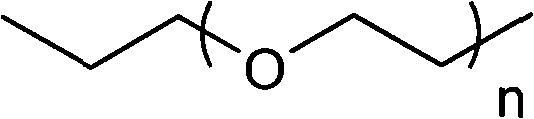
F-triazole ring-polyethylene glycol-metronidazole compound and preparation method thereof
A polyethylene glycol and compound technology, applied in the field of metronidazole compounds and their preparation, can solve the problems of low target/non-target ratio, long time interval between injection and imaging, slow removal rate of non-target tissue, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1- Embodiment 6
[0105] Embodiment 1-embodiment 6 ( 19 Synthesis of F)
Embodiment 1
[0107] 1. Synthesis of the Intermediate Alkyne PETY1
[0108] 1) The structure of the compound PETY1
[0109]
[0110] 2) Synthesis method
[0111] a. Synthesis of compound PEGY1
[0112] Under the protection of inert gas, 3-bromo-1-propyne (5 mL, 45 mmol) and 1,2-ethanediol (5.57 g, 90 mmol) were successively added into a dry round bottom flask. After stirring and mixing, ground and crushed NaOH (2.16g, 54mmol) was added in an ice-water bath, stirred rapidly for 15 minutes, then heated to 45°C, and reacted for 3 hours until the alkyne reaction was complete. After the reaction was completed, it was filtered, and the solid was washed with ethyl acetate. The filtrate was washed with a small amount of water until neutral. The filtrate was collected, washed successively with water and saturated brine, and finally dried over sodium sulfate. After filtering again, the organic solvent was removed under reduced pressure, followed by column chromatography. The product componen...
Embodiment 2
[0151] 1. Synthesis of the Intermediate Alkyne PETY2
[0152] 1) The structure of compound PETY2
[0153]
[0154] 2) Synthesis method
[0155] a. Synthesis of compound PEGY2
[0156] Under the protection of an inert gas, 3-bromo-1-propyne (5 mL, 45 mmol) and ethylene glycol (7.2 g, 68 mmol) were successively added into a dry round bottom flask. After stirring and mixing, ground NaOH (2.16 g, 54 mmol) was added in an ice-water bath, stirred, and reacted for 15 minutes. Then react at room temperature for 3 hours until the alkyne reaction is complete. Filter and rinse the solid with ethyl acetate. The filtrate was washed with a small amount of water until neutral. The filtrate was collected, washed successively with water and saturated brine, and finally dried over sodium sulfate. After filtering again, the organic solvent was removed under reduced pressure, followed by column chromatography. The product fractions were collected, and the solvent was removed under reduc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com