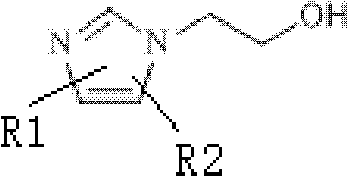
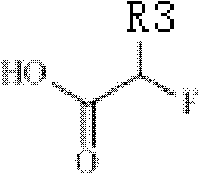
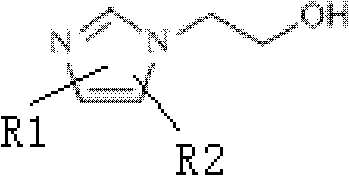
18/19F-ester nitroimidazole compound, preparation method thereof and application as hypoxic tissue developing agent
A technology of nitroimidazole and compound, applied in the field of radiopharmaceutical chemistry, can solve the problems of poor pharmacokinetic properties, poor metabolic stability and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Br-MNEFT, i.e. (2-(2-methyl-5-nitro-1-imidazolyl))ethyl 2-bromoacetate
[0057] Preparation of 2-(2-methyl-5-nitro-1H-imidazol-1-yl)ethyl 2-bromoacetate (compound 7 for short):
[0058]
[0059] 2-Methyl-5-nitroimidazole-1-ethanol (1.06mmol, 182mg) was dissolved in 10mL of dichloromethane, and triethylamine (0.6mL) and bromoacetyl bromide (2.87mmol, 579mg, 0.25mL), the reaction was stirred overnight. Then, the reaction mixture solution was diluted with 30 mL of dichloromethane, washed with water (10 mL×2) and saturated brine (10 mL). The resulting organic phase was dried over anhydrous sodium sulfate. After filtration, dichloromethane was distilled off under reduced pressure, and the obtained oil was separated by column chromatography using a silica gel column with ethyl acetate / n-hexane = 1 / 1 (v / v) as the eluent, and the product components were collected and distilled under reduced pressure. The solvent was removed to obtain 146 mg of compound 7 as a pale yellow ...
Embodiment 2
[0063] F-MNEFT, namely (2-(2-methyl-5-nitro-1-imidazolyl))ethyl 2-fluoroacetate
[0064] Preparation of 2-(2-methyl-5-nitro-1H-imidazol-1-yl)ethyl 2-fluoroacetate (compound 8 for short):
[0065]
[0066] 2-Methyl-5-nitroimidazole-1-ethanol (1.04mmol, 178mg) was dissolved in 10mL of dichloromethane, triethylamine (1.1mL) and fluoroacetyl chloride (2.23mmol, 193 mg, 0.17 mL). After 3 h the mixture was brought to room temperature and the reaction was stirred overnight. Then, the reaction mixture solution was diluted with 20 mL of dichloromethane, washed with water (10 mL×2) and saturated brine (10 mL). The resulting organic phase was dried over anhydrous sodium sulfate. After filtration, dichloromethane was distilled off under reduced pressure, and the obtained oil was separated by column chromatography using a silica gel column with ethyl acetate / n-hexane = 1 / 1 (v / v) as the eluent, and the product components were collected and distilled under reduced pressure. The solven...
Embodiment 3
[0070] Br-NPFT, namely (2-(2-nitro-1-imidazolyl))ethyl 2-bromopropionate
[0071] Preparation of 2-(2-nitro-1H-imidazol-1-yl)ethyl 2-bromopropanoate (compound 9 for short):
[0072]
[0073] 2-Nitroimidazole-1-ethanol (1.03mmol, 162mg) was dissolved in 6mL of dichloromethane, triethylamine (0.4mL) was added dropwise in an ice-water bath, and bromine diluted with 2mL of dichloromethane was added dropwise. Propionyl bromide (0.2 mL), the reaction was stirred overnight. Then, the reaction mixture solution was diluted with 10 mL of dichloromethane, washed with water (5 mL×2) and saturated brine (5 mL). The obtained organic phase was dried over anhydrous sodium sulfate. After filtration, dichloromethane was distilled off under reduced pressure, and the resulting oil was separated by column chromatography using a silica gel column with ethyl acetate / n-hexane=2 / 3 (v / v) as the eluent, and the product components were collected and distilled under reduced pressure The solvent was ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com