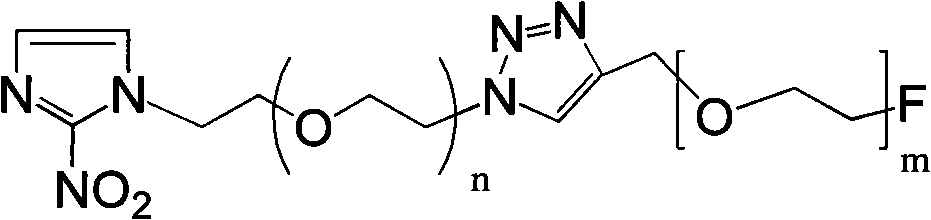
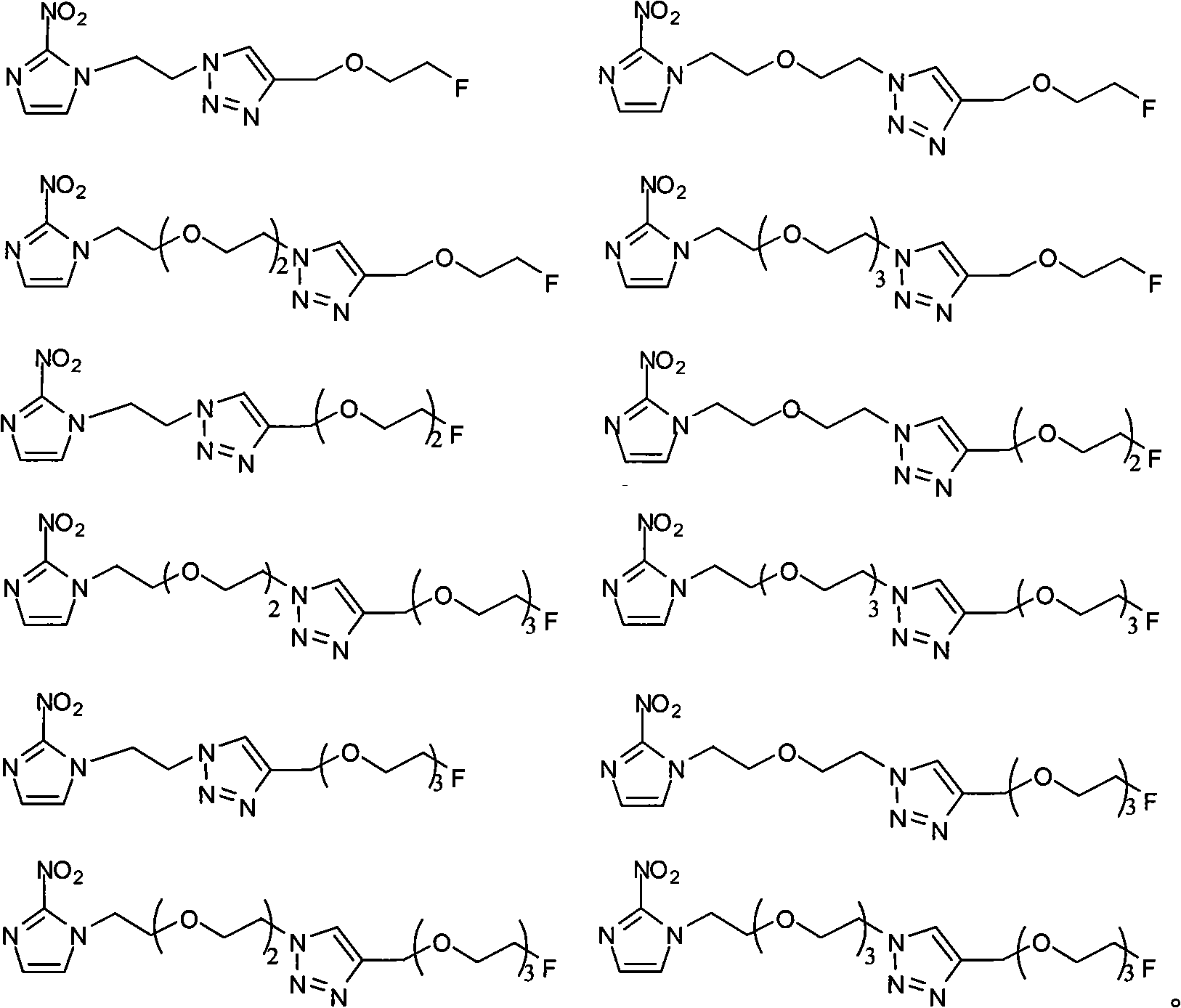
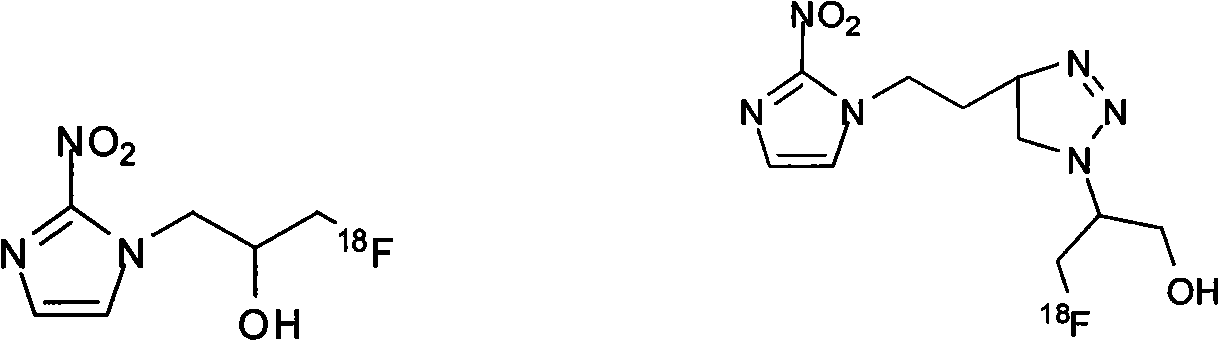
Novel F-triazole ring-polyethyleneglycol-2-nitroimidazole compound and preparation method thereof
A technology of polyethylene glycol and nitroimidazole, which is applied in the field of nitroimidazole compounds and their preparation, and can solve problems such as the reporting of research results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1- Embodiment 4
[0060] Embodiment 1-embodiment 4 ( 19 Synthesis of F)
Embodiment 1
[0062] 1. Synthesis of Intermediate Alkynes
[0063] Synthesis of PETY-1 (m=1)
[0064] (1) The structure of compound PETY-1
[0065]
[0066] (2) Synthesis method
[0067] a. Synthesis of PEGY-1
[0068] Add propyne bromide (5.34g, 45mmol) and ethylene glycol (5.57g, 20mmol) into a 25mL three-necked flask under the protection of argon, and slowly add NaOH into the reaction flask under stirring in an ice bath, and keep the ice-water bath for 15min. Then heated to reflux at 40°C for 3h. After the reaction was completed, it was filtered, and the solid was washed with ethyl acetate. The filtrate was washed with a small amount of water until neutral. Na for organic phase 2 SO 4 Dry, filter, remove the solvent under reduced pressure, and perform column chromatography, the solvent ratio is ethyl acetate:n-hexane=1:3. The product fractions were collected, and the solvent was removed under reduced pressure to obtain 1.50 g of a yellow oil, with a yield of 34.2%.
[0069] ...
Embodiment 2
[0097] The synthesis of the intermediate alkyne is the same as in Example 1.
[0098] Synthesis of Compound NIA2-TAF (n=1, m=1)
[0099] (1) The structure of the compound NIA2-TAF
[0100]
[0101] (2) Synthesis method
[0102] a. Synthesis of Compound PETA-2
[0103]Ethylene glycol (4.24 g, 40 mmol, 3.9 mL) was dissolved in 30 mL of dichloromethane. Under the condition of ice-water bath, p-toluenesulfonyl chloride (18.30g, 96mmol) was added to the above solution, and then sodium hydroxide (6.40g, 160mmol) was added slowly, and the reaction was stirred at 0-5°C for 2-3h in ice-water bath. After the reaction was completed, 50 mL of dichloromethane and 30 mL of ice water were added, shaken, and separated into layers. The organic phase was washed successively with hydrochloric acid solution, saturated sodium bicarbonate solution, and ice water until neutral, Na 2 SO 4 After drying, the solvent was removed under reduced pressure to obtain a crude product. The crude produ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com