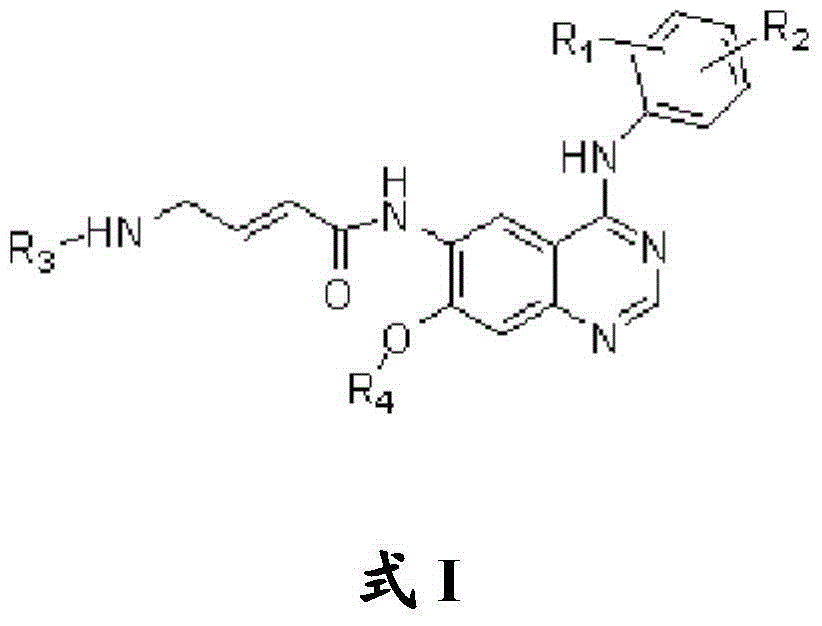
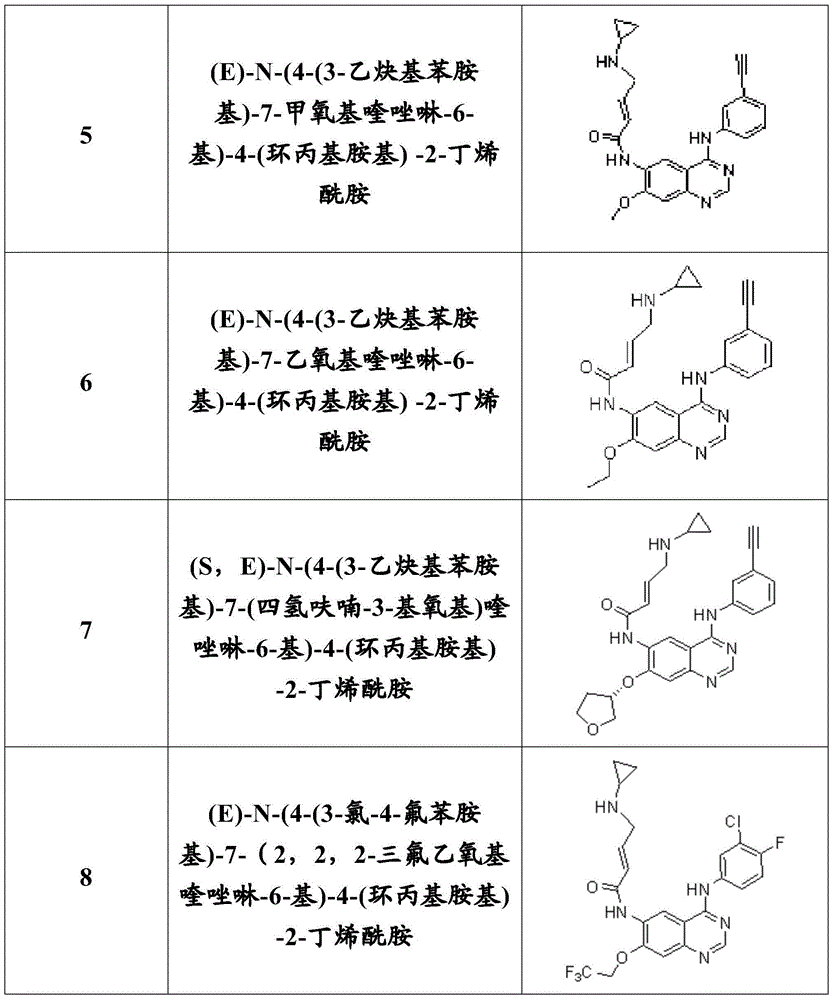
Quinazoline compound, preparation method and application thereof
A compound, selected technology, applied in the fields of organic chemistry, drug combination, anti-tumor drugs, etc., can solve the problems of prolonging the overall survival of patients, difficult to take effect, and no observed therapeutic effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
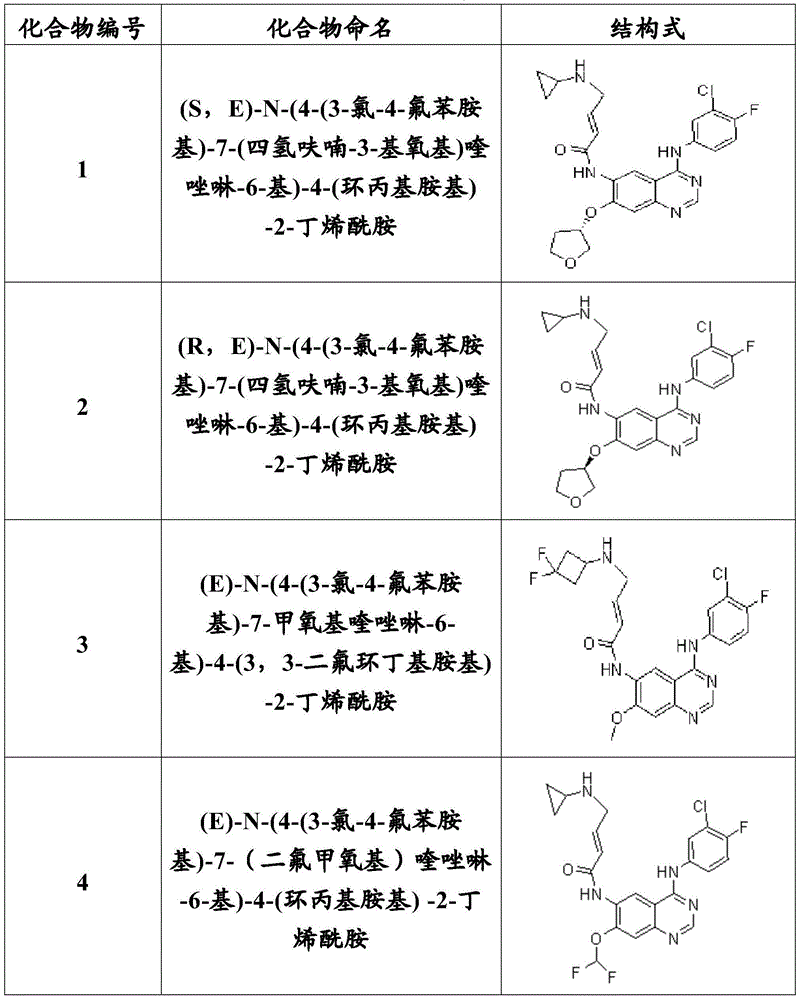
[0102] Example 1: (S, E)-N-(4-(3-chloro-4-fluoroanilino)-7-(tetrahydrofuran-3-yloxy) Synthesis of quinazolin-6-yl)-4-(cyclopropylamino)-2-butenamide (compound 1)
[0103]
[0104] a. Synthesis of N-(3-chloro-4-fluorophenyl)-6-nitro-7-fluoroquinazolin-4-amine
[0105]
[0106]Take 4-hydroxy-7-fluoro-6-nitroquinazoline (20g) into a 1000ml single-necked bottle, add 390ml of chloroform (W / V=1:19.5), and start to cool down to below 5°C under stirring . The reaction temperature was controlled below 5°C, and 24.5g (2eq) of oxalyl chloride was added dropwise with stirring. After dropping, control the internal temperature below 5°C, and drop DMF in chloroform solution (2.4ml dissolved in 100ml chloroform) under stirring. After dropping, the temperature of the reaction began to rise, and the reaction was stirred in an oil bath at 70° C. (reflux), followed by TLC until the reaction was completed, and the temperature was lowered to 20° C. A mixture of anhydrous magnesium su...
Embodiment 2
[0118] Example 2: (R, E)-N-(4-(3-chloro-4-fluoroanilino)-7-(tetrahydrofuran-3-yloxy) Synthesis of quinazolin-6-yl)-4-(cyclopropylamino)-2-butenamide (compound 2)
[0119]
[0120] In a similar manner to the preparation of compound 1, compound 2 (19% yield) could be obtained as a khaki granular solid using (R)-3-hydroxytetrahydrofuran as a starting material.
[0121] ESI-MS (m / z): [M+H] + 498.2.
Embodiment 3
[0122] Example 3: (E)-N-(4-(3-chloro-4-fluoroanilino)-7-methoxyquinazolin-6-yl)-4-(3, Synthesis of 3-difluorocyclobutylamino)-2-butenamide (Compound 3)
[0123]
[0124] Similar to the preparation of compound 1, using methanol and 3,3-difluorocyclobutylamine as starting materials, compound 3 (13% yield) could be obtained as a yellow solid.
[0125] 1 H-NMR (600MHz, DMSO-d 6 ,δ ppm ): 9.81(s, 1H), 9.65(s, 1H), 8.92(s, 1H), 8.34(s, 1H), 8.13(dd, 1H, J 1 =7.2Hz,J 2 =3.0Hz), 7.80(m, 1H), 7.43(t, 1H), 7.29(s, 1H), 6.85(m, 1H), 6.56(d, 1H, J=15.0Hz), 4.04(s, 3H ), 3.30(d, 2H), 3.15(m, 1H), 2.75(m, 2H), 2.34(m, 2H).
[0126] ESI-MS (m / z): [M+H] + 492.4.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com