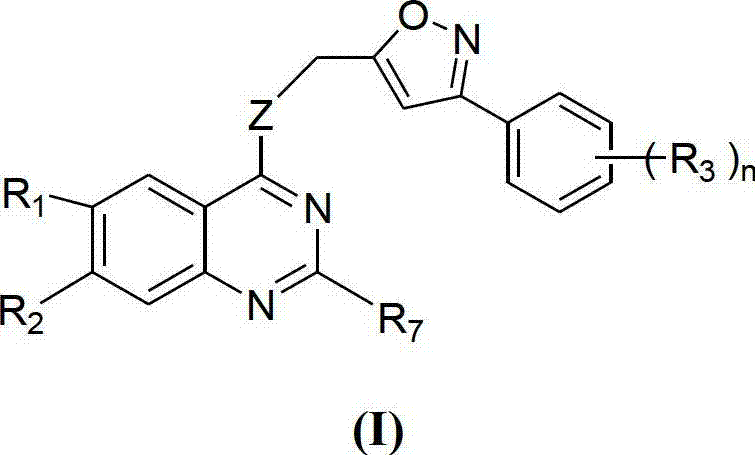
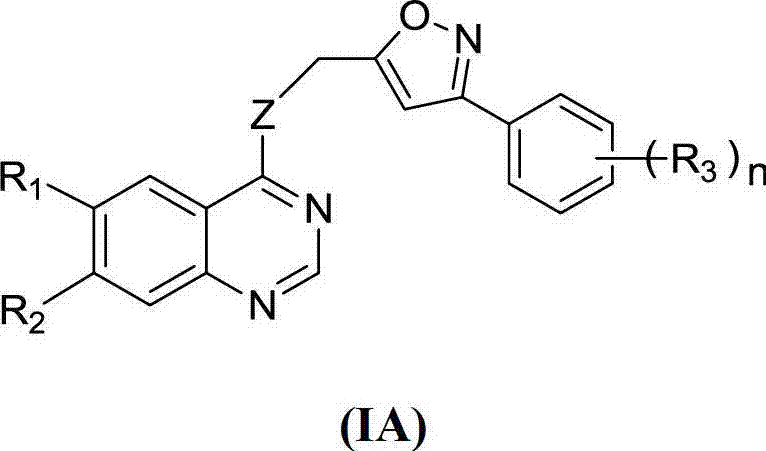
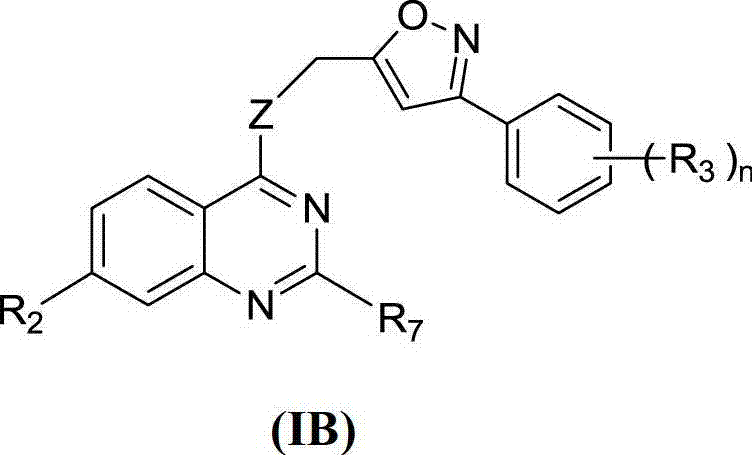
Quinazoline derivative and application thereof
A technology of quinazoline and isoxazole, which is applied in the field of quinazoline derivatives and applications, and can solve problems such as uncontrollable cell division
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 16
[0242] The synthesis of embodiment 16,7-dimethoxy-4-chloro-quinazoline
[0243] Add 4.12g (20mmol) of 6,7-dimethoxy-quinazolinone into a 500mL single-necked round low flask, then slowly add 120mL of redistilled thionyl chloride containing 1 drop of DMF, reflux reaction, TLC detection After the reaction was completed, excess thionyl chloride was distilled off under reduced pressure, and the residue was dissolved in 300 mL of ethyl acetate, washed with saturated sodium bicarbonate solution to neutrality, and the organic layer was dried over anhydrous sodium sulfate, concentrated and separated by column (V 石油醚 :V 乙酸乙酯 :4:1~2:1) to get 6,7-dimethoxy-4-chloro-quinazoline, yield: 85%, 1 H NMR (DMSO-d 6 ,400MHz):4.01(s,6H,2CH 3 ),7.38(s,1H),7.45(s,1H),8.88(s,1H);ESI-MS(100%):224([M] + ,100).
Embodiment 27
[0244] The synthesis of embodiment 27-nitro-quinazolone
[0245] Add 46g (0.25mol) of 2-amino-4-nitrobenzoic acid and 52g (0.5mol) of formamidine acetate into a 500mL single-necked round-bottomed flask, add 200mL of absolute ethanol, and reflux for 4 to 6 hours. There will be A large number of khaki precipitates. Stop heating at this time, cool the system to about 50°C, remove part of the ethanol in vacuum to about 50mL of the system, add 30mL of water to the system, stir the system for a few minutes, refrigerate for 1h, filter, and filter the cake with refrigerated ethanol (3×10mL) Washing, washing with water (2×10mL), drying in vacuo to give 7-nitro-quinazolinone 2, 39.6g khaki needle-like solid, yield: 82.8%, m.p: 282~284°C, IR(KBr) ν: 3174, 3064, 2960, 1683, 1613, 1528, 803; this compound can be directly used in the next chlorine substitution reaction without further purification.
Embodiment 37
[0246] The synthesis of embodiment 37-nitro-4-chloro-quinazoline
[0247] Take 19.1g (0.1mol) of 7-nitro-quinazolinone in a 250mL three-neck flask, slowly add 60mL of freshly steamed SOCl containing 2 drops of DMF dropwise under stirring 2 , reflux reaction after adding, TLC monitors the progress of the reaction, after the reaction is completed, excess SOCl is evaporated under reduced pressure 2 , the residue was washed with refrigerated ether (5×20mL) to obtain the crude product, and the crude product was recrystallized with petroleum ether and ethyl acetate to obtain 19.0g of the compound intermediate 7-nitro-4-chloro-quinazoline, white needles shape crystal. Yield: 90.6%, m.p: 150~151℃, 1 H NMR (CDCl 3 ,400MHz)δ:8.55(s,2H,Ph-H),8.98(s,1H,Ph-H),9.23(s,1H,Ar-H);IR(KBr)ν:3057,2959,1528, 1469, 1357, 805, 743.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com