Preparation method of transferrin, transferrin-containing composition and application
A technology for transferrin and a composition is applied in the field of preparation of transferrin, which can solve the problems of complex plasma composition, and achieve the effects of improving treatment effect, improving self-care ability and enhancing memory.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
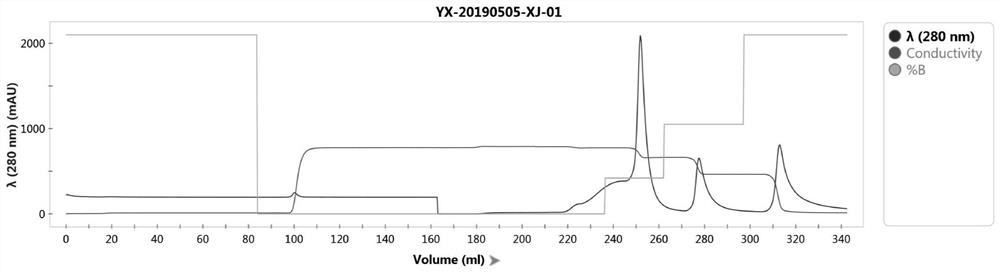
[0067] The specific preparation method of the above-mentioned transferrin is as follows: the following steps are sequentially included: preparation of pretreatment solution, purification by hydrophobic chromatography, concentration and replacement.
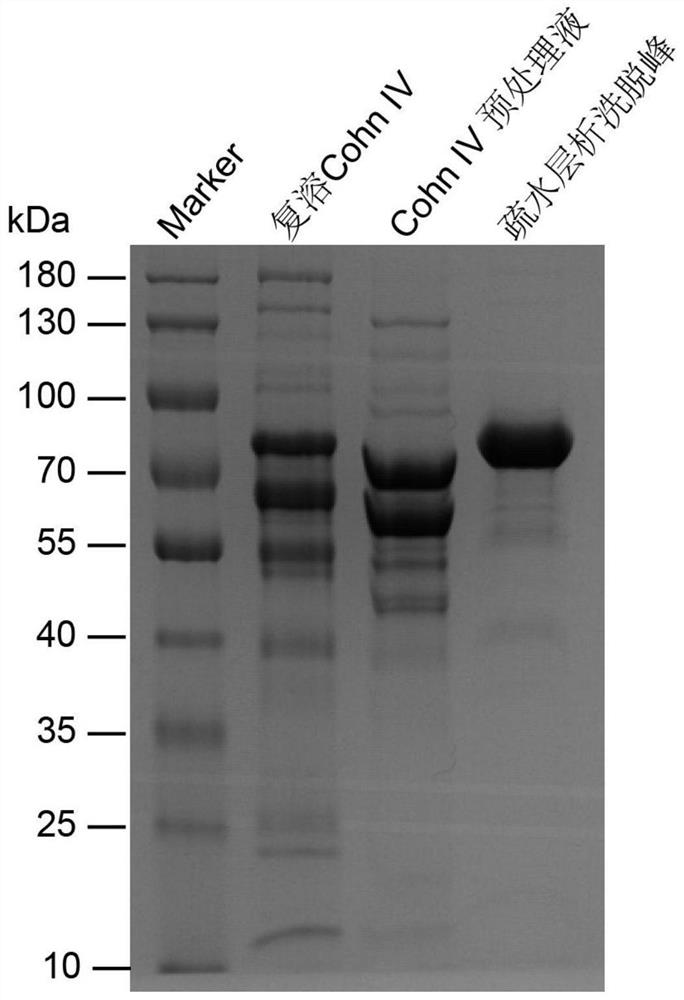
[0068] Step 1. Preparation of pretreatment solution: the purpose of this step is to redissolve the protein in the precipitated human plasma Cohn IV fraction, remove the filter aid, and remove some miscellaneous proteins after reconstitution.
[0069] Sub-step 1, the first reconstitution treatment: dissolve the solid Cohn IV component into PBS buffer, mix well, and obtain the first reconstitution solution.
[0070] The mass volume ratio (w / v) of above-mentioned human plasma Cohn IV component and PBS buffer is 1: (2-10), preferably 1: 3.5; Above-mentioned PBS buffer is: 10-50mmol / L PBS, pH6. 0-8.0, preferably 20mmol / L PBS, pH7.5.
[0071] Sub-step 2, the first centrifugation treatment: centrifuge the above-mentioned first complex s...
Embodiment 1
[0098] The present embodiment is the preparation method of transferrin, comprising the following steps:
[0099] S1: Preparation of human plasma Cohn IV fraction pretreatment solution:
[0100] (1) The first reconstitution: Dissolve the waste material from the production of blood products by the Nitschmann-Kistler method—the human plasma Cohn IV component solid into 20mmol / L PBS solution (pH7.5), according to 1:3.5 (w / v) After dissolving, use a magnetic stirrer to mix evenly to obtain the first complex solution.
[0101] (2) The first centrifugation: centrifuge the first complex solution at 10,000 rpm at 4° C. to obtain the first supernatant.
[0102] (3) Filtration for the first time: filter the supernatant for the first time with a 0.45 μm filter membrane to obtain the filtrate for the first time.
[0103] (4) pH adjustment: adjust the pH value of the first filtrate to 7.0 with 1 mol / L sodium citrate.
[0104] (5) Remove impurity protein: Add 15% (w / v) PEG4000 to the fir...
Embodiment 2
[0123] In this example, the sterilized transferrin (TF solution) obtained in step S4 of Example 1 is used in combination with human serum albumin (HSA) and human immunoglobulin (IgG).
[0124] The sterilized transferrin was mixed with human serum albumin and human immunoglobulin to obtain a TF+HSA+IgG mixed solution, and the mass ratio of TF, HSA and IgG in the mixed solution was 1:8.33:8.33.
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com