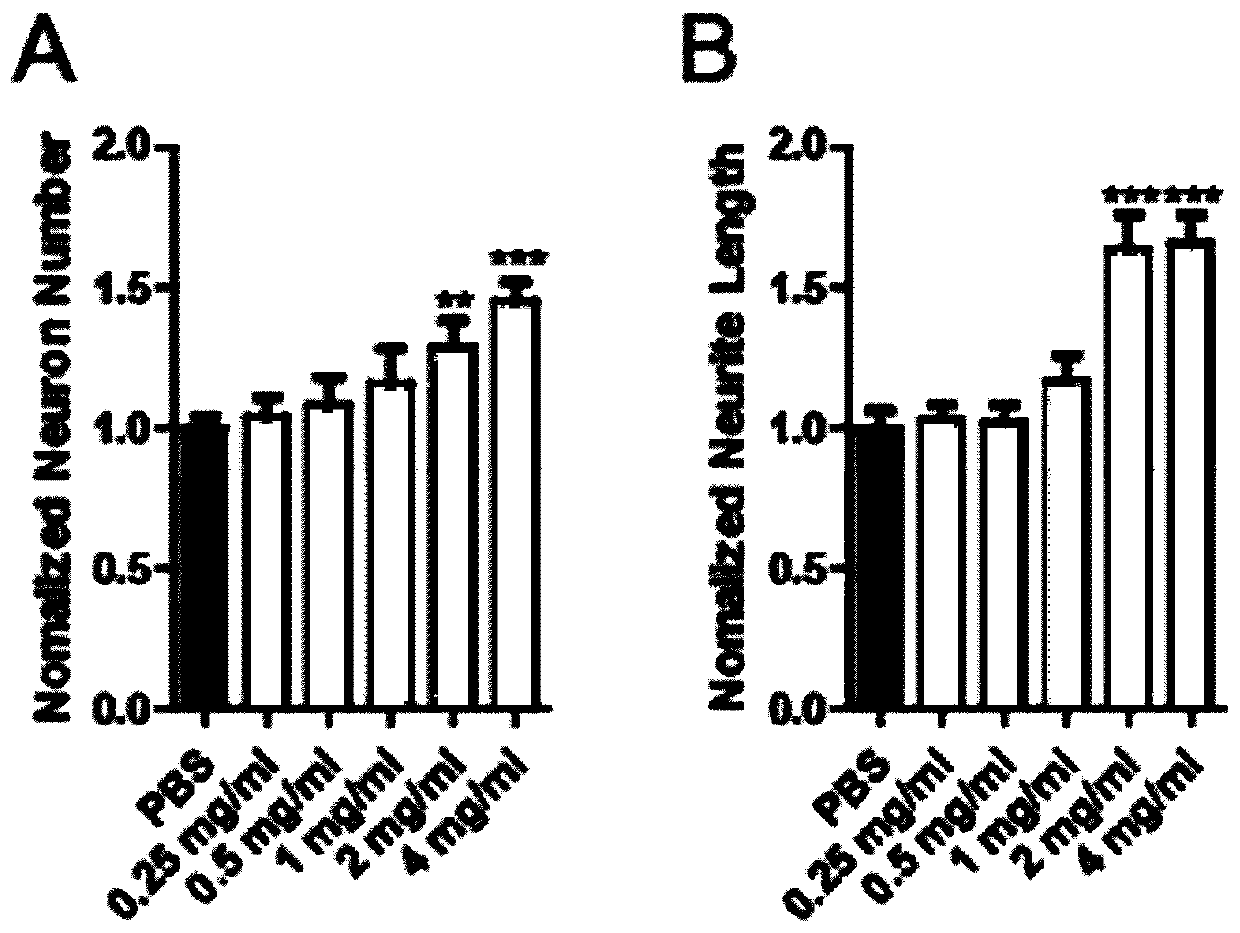
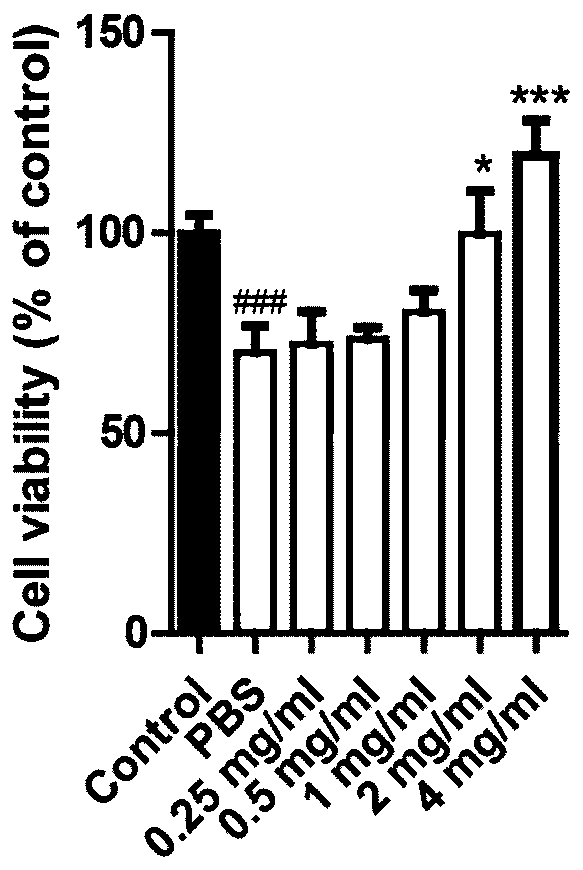
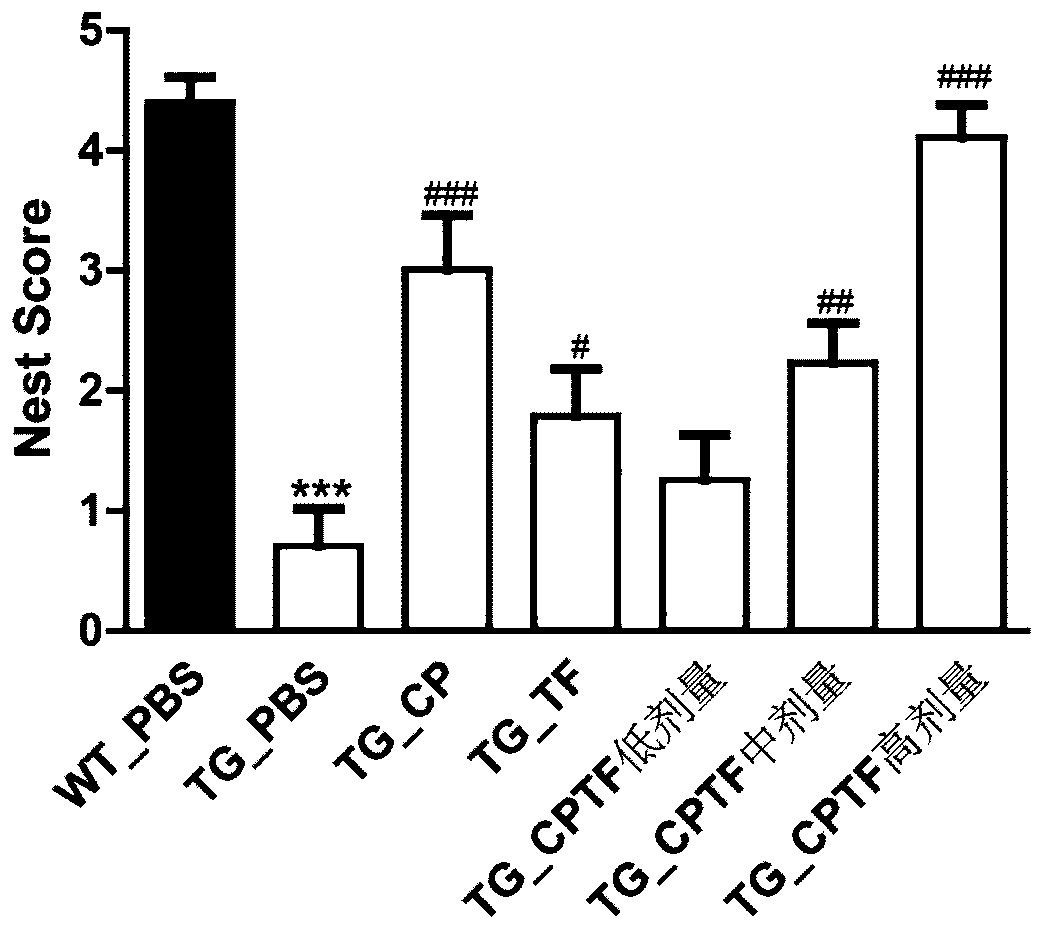
Application of union of covelline albumen and transferrin
A technology of ceruloplasmin and transferrin, applied in the direction of transferrin, peptide/protein components, animal/human protein, etc., to achieve the effect of improving cognitive function, learning and memory ability, and self-care ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0039] The specific preparation method of the above-mentioned transferrin is as follows: the following steps are sequentially included: preparation of pretreatment solution, purification by hydrophobic chromatography, concentration and replacement.
[0040] Step 1. Preparation of pretreatment solution: the purpose of this step is to redissolve the protein in the precipitated human plasma Cohn IV fraction, remove the filter aid, and remove some miscellaneous proteins after reconstitution.
[0041] Sub-step 1, the first reconstitution treatment: dissolve the solid Cohn IV component into PBS buffer, mix well, and obtain the first reconstitution solution.
[0042] The mass volume ratio (w / v) of above-mentioned human plasma Cohn IV component and PBS buffer is 1: (2-10), preferably 1: 3.5; Above-mentioned PBS buffer is: 10-50mmol / L PBS, pH6. 0-8.0, preferably 20mmol / L PBS, pH7.5.
[0043] Sub-step 2, the first centrifugation treatment: centrifuge the above-mentioned first complex s...
Embodiment 1
[0071] The present embodiment is the preparation method of transferrin, comprising the following steps:
[0072] S1: Preparation of human plasma Cohn IV fraction pretreatment solution:
[0073] (1) The first reconstitution: Dissolve the waste material from the production of blood products according to the Nitschmann-Kistler method—the human plasma Cohn IV component solid into 20mmol / L PBS solution (pH7.5), according to the ratio of 1:3.5 (w / v) After dissolving, use a magnetic stirrer to mix evenly to obtain the first complex solution.
[0074] (2) The first centrifugation: centrifuge the first complex solution at 10,000 rpm at 4° C. to obtain the first supernatant.
[0075] (3) Filtration for the first time: filter the supernatant for the first time with a 0.45 μm filter membrane to obtain the filtrate for the first time.
[0076] (4) pH adjustment: adjust the pH value of the first filtrate to 7.0 with 1 mol / L sodium citrate.
Embodiment 2
[0096] This embodiment is the combined use of ceruloplasmin and transferrin.
[0097] For the same experimental animal, in the same injection of the ceruloplasmin+transferrin composition, the mass ratio of ceruloplasmin to transferrin is 1:1. Among them, the activity of ceruloplasmin is 1.02unit / mg, and the purity of transferrin is 95%.
[0098] The aforementioned transferrin is the sterilized transferrin (TF solution) obtained in Step S4 of Example 1. The above-mentioned ceruloplasmin is obtained with reference to the method on pages 17-32 of the above-mentioned thesis. The pig serum in 3.1.5.1 is replaced with the serum of a healthy person to obtain a dialysate; described), hydroxyapatite adsorption chromatography (as described in 3.1.5.3), and Superdex-200 gel filtration chromatography (as described in 3.1.5.4), the component with the highest specific activity was used as ceruloplasmin Pure. Then, the pure ceruloplasmin was sterilized by filtration, the filter membrane w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com