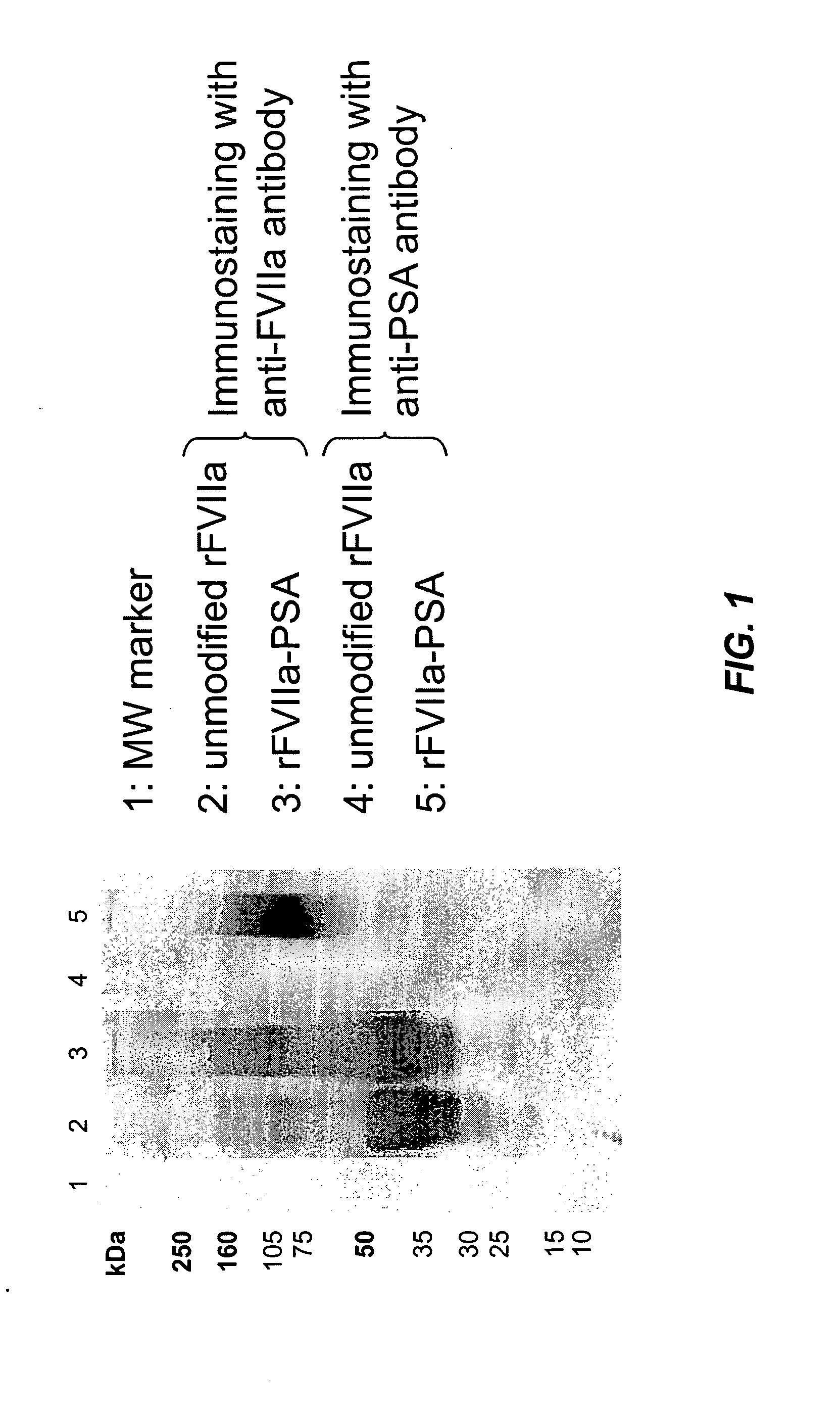
Patents
Literature
688 results about "Factor XIIIa" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Activated form of FACTOR XIII, a transglutaminase, which stabilizes the formation of the fibrin polymer (clot) culminating the blood coagulation cascade.
Particulate acellular tissue matrix
A method of processing an acellular tissue matrix to give a particulate acellular tissue matrix includes: cutting sheets of dry acellular tissue matrix into strips; cryofracturing the dry acellular tissue matrix strips at cryogenic temperatures; separating the resulting particles by size at cryogenic temperatures; and freeze drying the fraction of particles desired size to remove any moisture that may have been absorbed to give a dry particulate acellular tissue matrix. Rehydration of the dry particulate acellular tissue matrix may take place just prior to use. The particulate acellular tissue may be applied to a recipient site, by way of injection, spraying, layering, packing, in-casing or combinations thereof. The particulate acellular tissue may further include growth and stimulating agents selected from epidermal growth factor, fibroblast growth factor, nerve growth factor, keratinocyte growth factor, platelet derived growth factor, vasoactive intestinal peptide, stem cell factor, bone morphogetic proteins, chondrocyte growth factor and combinations thereof. Other pharmaceutically active compounds may be combined with the rehydrated particulate material including: analgesic drugs; hemostatic drugs; antibiotic drugs; local anesthetics and the like to enhance the acceptance of the implanted particulate material. The particulate material product may also be combined with stem cells selected from mesenchymal stem cells, epidermal stem cells, cartilage stem cells, hematopoietic stem cells and combinations thereof.
Owner:LIFECELL
Formulations for factor IX
InactiveUS6372716B1Pharmaceutical delivery mechanismSaccharide peptide ingredientsFactor iiFactor IX
Owner:GENETICS INST INC
Coagulation factor VIIa composition
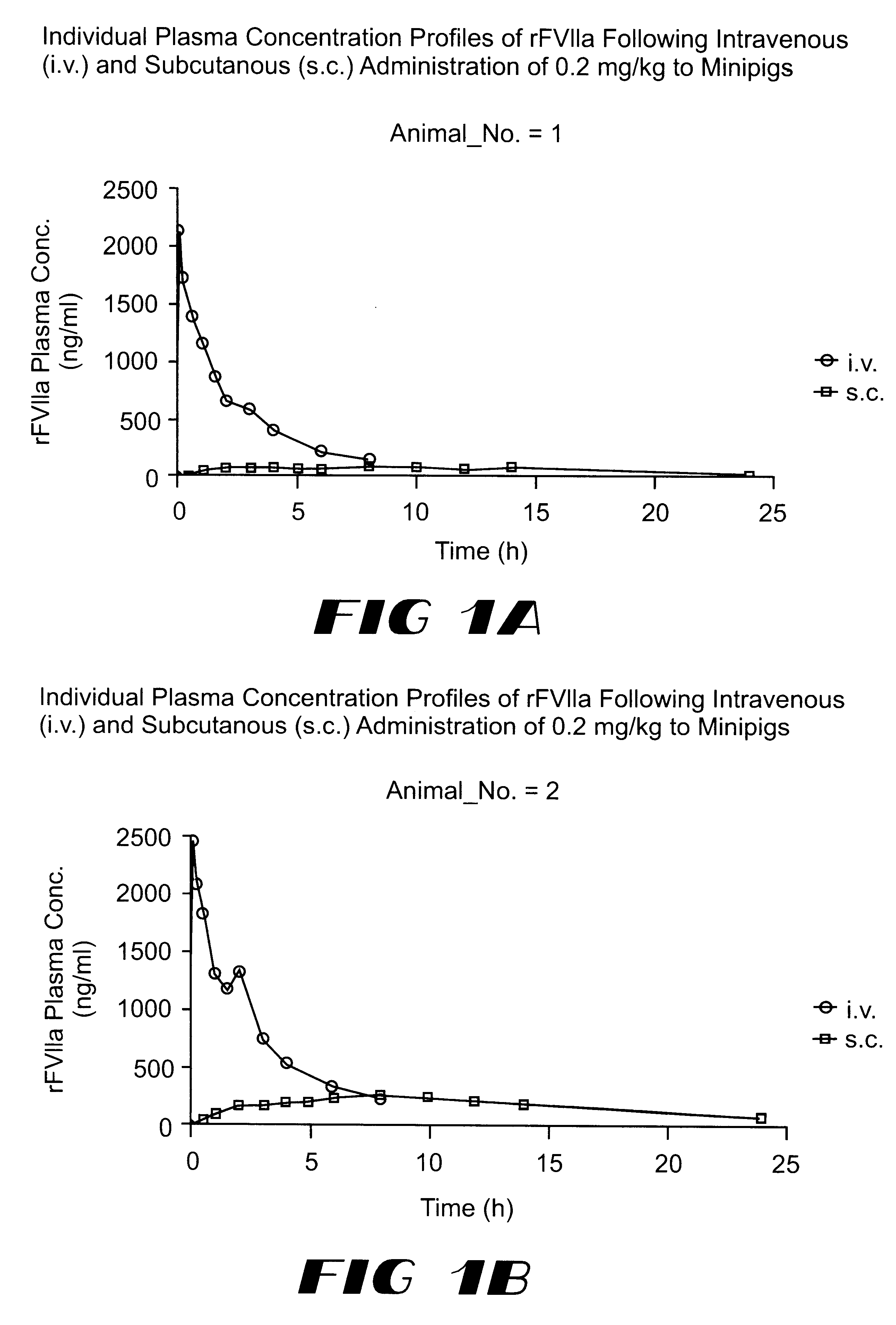
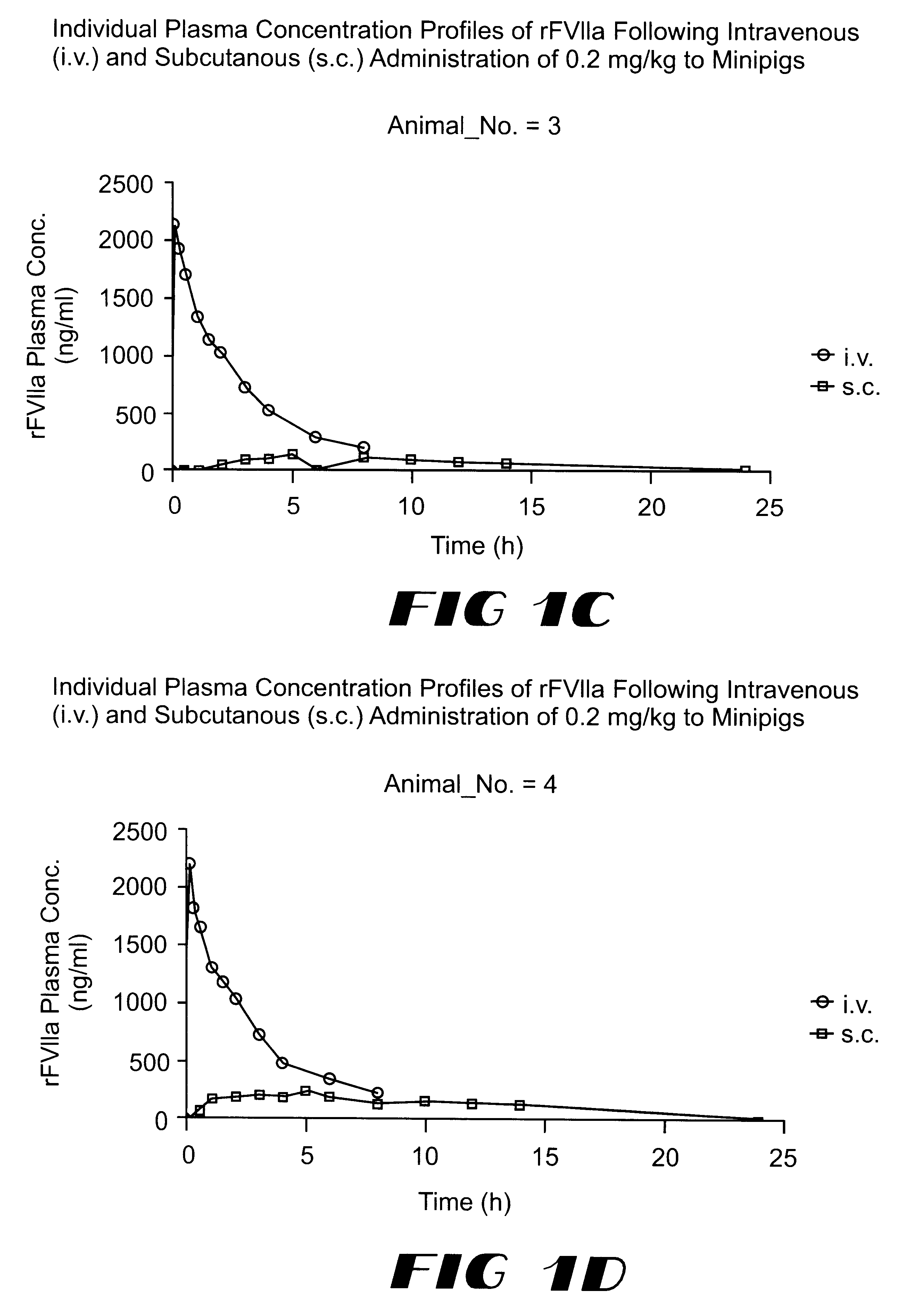
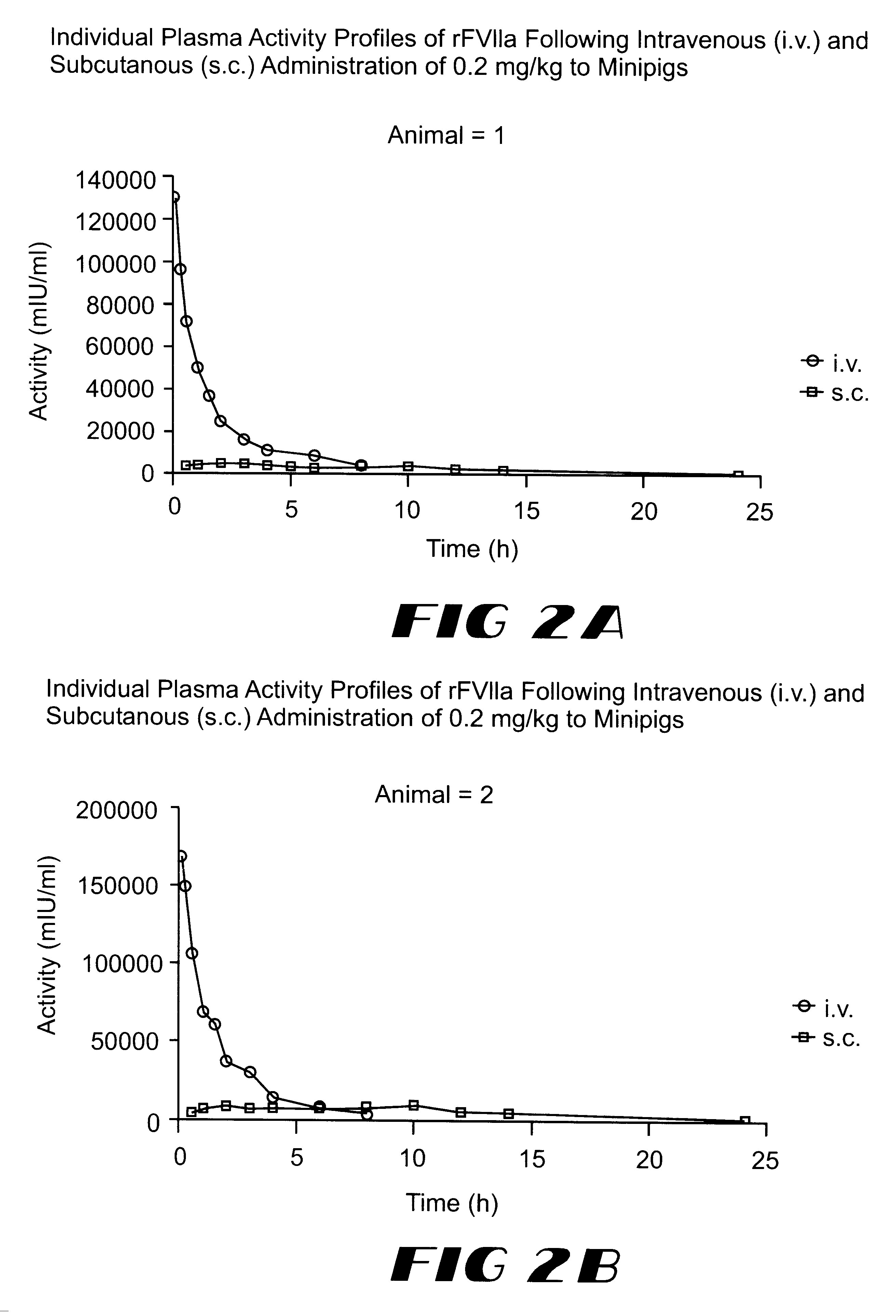
The invention relates to a pharmaceutical composition comprising Factor VIIa for subcutan, intramuscular or interdermal administration.Factor VIIa administered subcutanously, intramuscularly or intradermally shows a sufficient transport into the bloodstream in biologically active form and in adequate concentrations, and favorable pharmacokinetic properties.
Owner:NOVO NORDISK AS
Enzyme-mediated modification of fibrin for tissue engineering
The invention provides fibrin-based, biocompatible materials useful in promoting cell growth, wound healing, and tissue regeneration. These materials are provided as part of several cell and tissue scaffolding structures that provide particular application for use in wound-healing and tissue regenerating. Methods for preparing these compositions and using them are also disclosed as part of the invention. A variety of peptides may be used in conjunction with the practice of the invention, in particular, the peptide IKVAV, and variants thereof. Generally, the compositions may be described as comprising a protein network (e.g., fibrin) and a peptide having an amino acid sequence that comprises a transglutaminase substrate domain (e.g., a factor XIIIa substrate domain) and a bioactive factor (e.g., a peptide or protein, such as a polypeptide growth factor), the peptide being covalently bound to the protein network. Other applications of the technology include their use on implantable devices (e.g., vascular graphs), tissue and cell scaffolding. Other applications include use in surgical adhesive or sealant, as well as in peripheral nerve regeneration and angiogenesis.
Owner:CALIFORNIA INST OF TECH
Compositions and methods for adoptive and active immunotherapy
ActiveUS20100284965A1Easy modular assemblyHigh densityPowder deliveryBiocideControl mannerMicroparticle
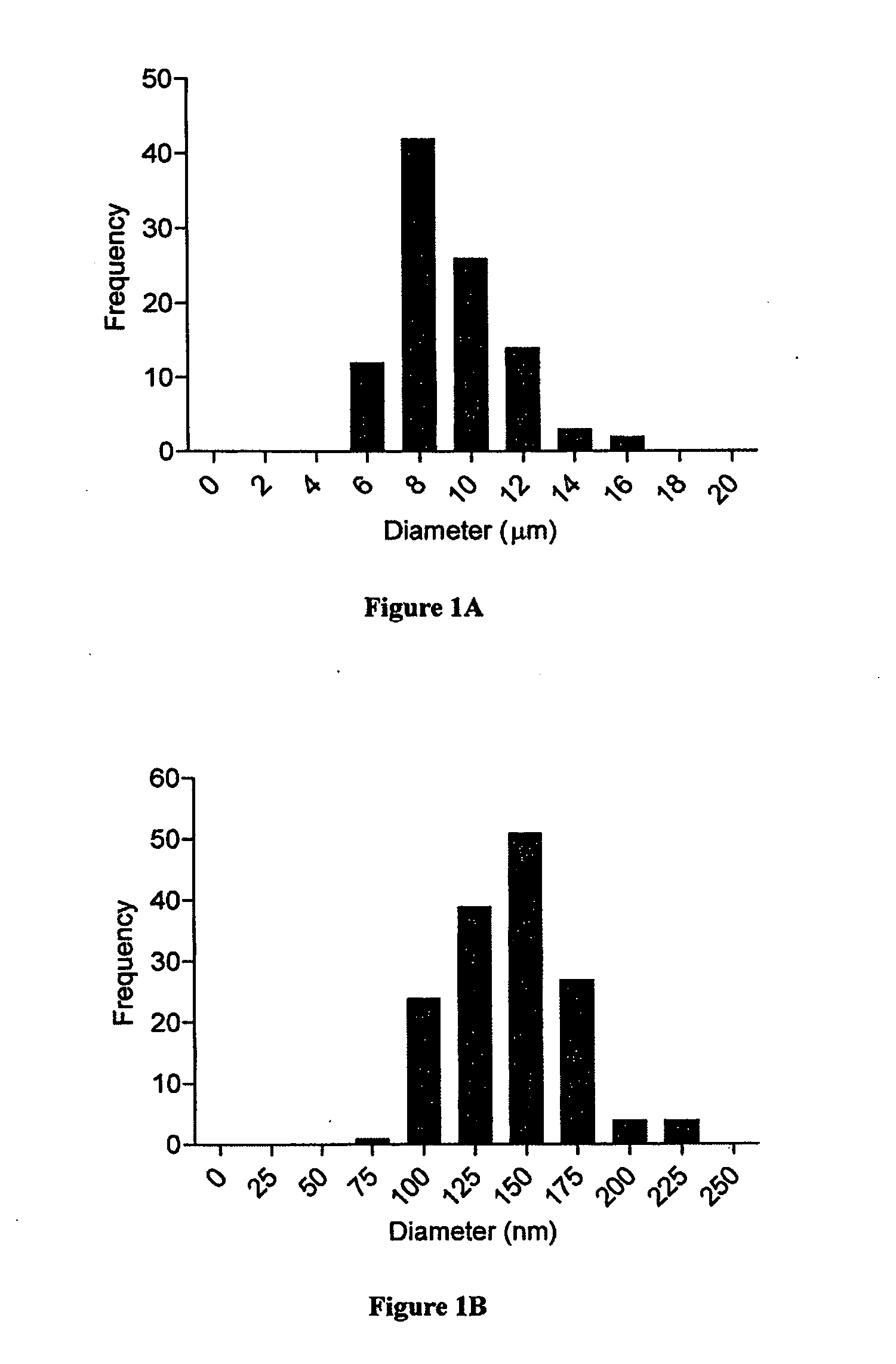
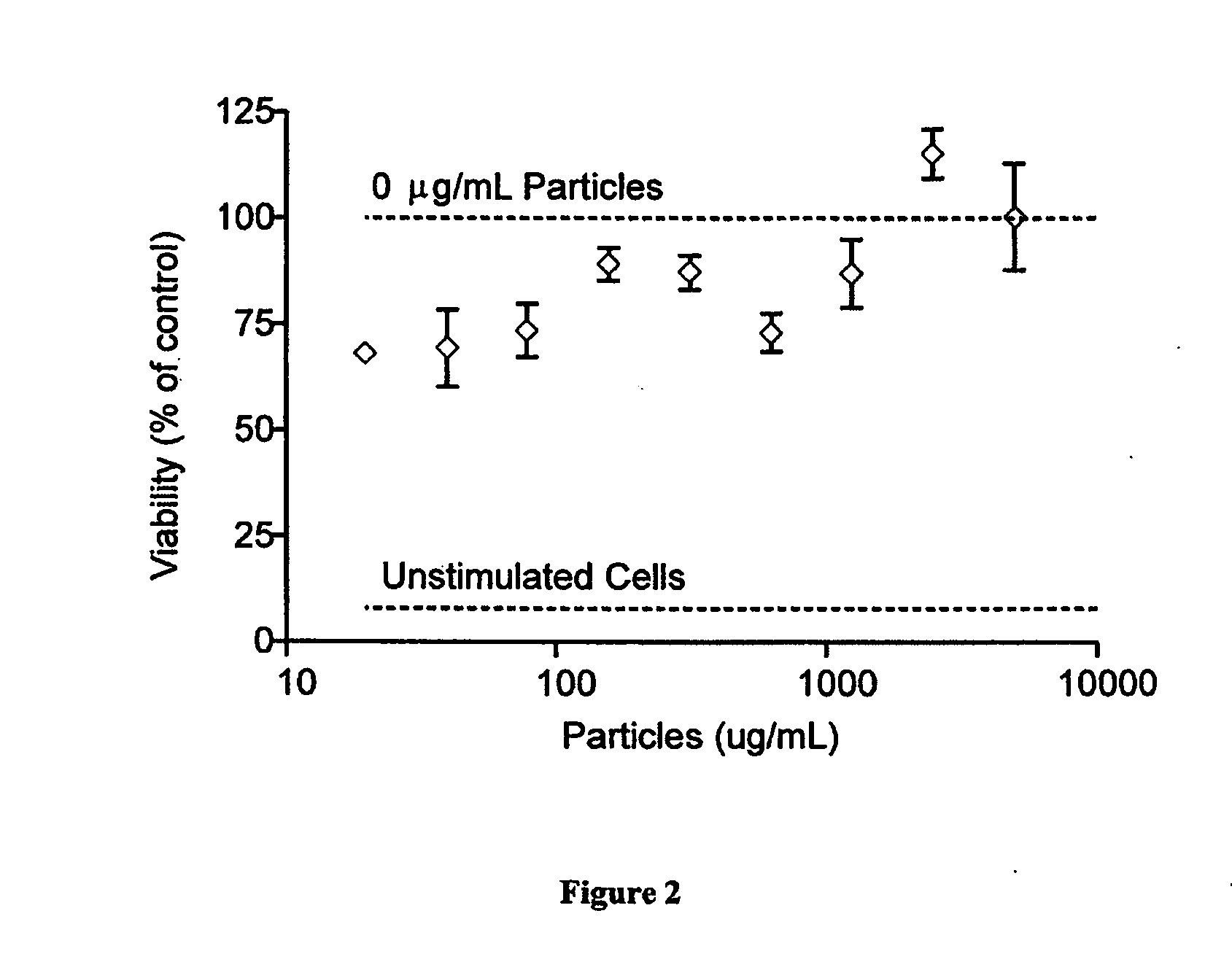
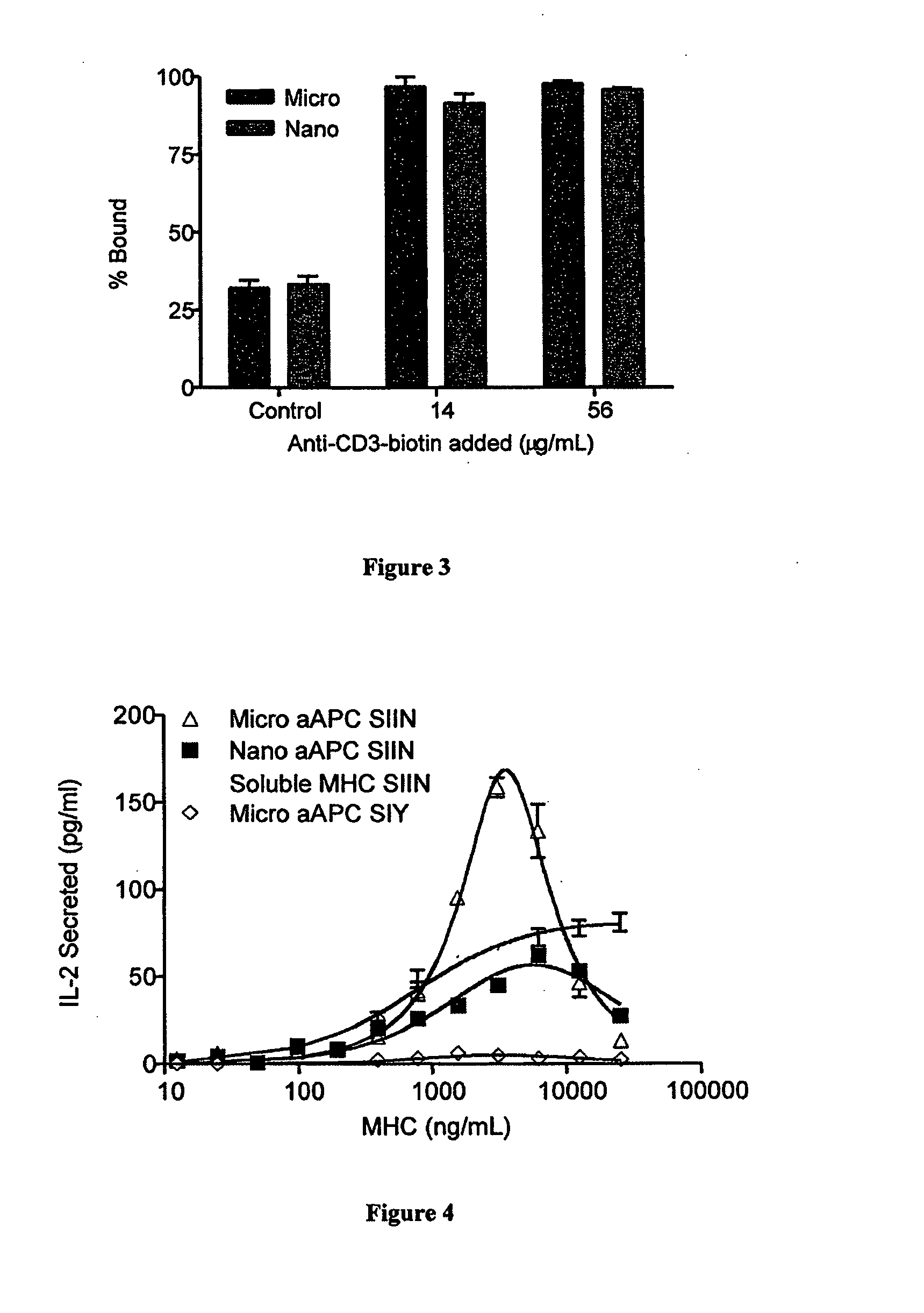
Modular aAPCs and methods of their manufacture and use are provided. The modular aAPCs are constructed from polymeric microparticles. The aAPCs include encapsulated cytokines and coupling agents which modularly couple functional elements including T cell receptor activators, co-stimulatory molecules and adhesion molecules to the particle. The ability of these aAPCs to release cytokines in a controlled manner, coupled with their modular nature and ease of ligand attachment, results in an ideal, tunable APC capable of stimulating and expanding primary T cells.
Owner:YALE UNIV
Bioabsorbable polymeric implants and a method of using the same to create occlusions
InactiveUS20020040239A1Peptide/protein ingredientsPharmaceutical containersPoly-L-lactideVascular compartment
A new embolic agent, bioabsorbable polymeric material (BPM) is incorporated to a Guglielmi detachable coil (GDC) to improve long-term anatomic results in the endovascular treatment of intracranial aneurysms. The embolic agent, comprised at least in part of at least one biocompatible and bioabsorbable polymer and growth factors, is carried by hybrid bioactive coils and is used to accelerate histopathologic transformation of unorganized clot into fibrous connective tissue in experimental aneurysms. An endovascular cellular manipulation and inflammatory response are elicited from implantation in a vascular compartment or any intraluminal location. Thrombogenicity of the biocompatible and bioabsorbable polymer is controlled by the composition of the polymer. The coil further is comprised at least in part of a growth factor or more particularly a vascular endothelial growth factor, a basic fibroblast growth factor or other growth factors. The biocompatible and bioabsorbable polymer is in the illustrated embodiment at least one polymer selected from the group consisting of polyglycolic acid, poly~glycolic acid / poly-L-lactic acid copolymers, polycaprolactive, polyhydroxybutyrate / hydroxyvalerate copolymers, poly-L-lactide. Polydioxanone, polycarbonates, and polyanhydrides.
Owner:RGT UNIV OF CALIFORNIA
Six-membered heterocycles useful as serine protease inhibitors
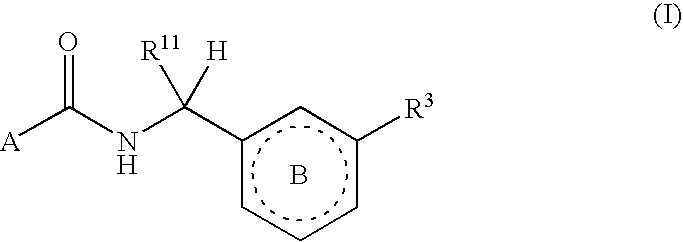
The present invention provides compounds of Formula (I):or a stereoisomer, tautomer, pharmaceutically acceptable salt or solvate form thereof, wherein the variables A, B, R3 and R11 are as defined herein. The compounds of Formula (I) are useful as selective inhibitors of serine protease enzymes of the coagulation cascade and / or contact activation system; for example thrombin, factor Xa, factor XIa, factor IXa, factor VIIa and / or plasma kallikrein. In particular, it relates to compounds that are selective factor XIa inhibitors or dual inhibitors of fXIa and plasma kallikrein. This invention also relates to pharmaceutical compositions comprising these compounds and methods of treating thromboembolic and / or inflammatory disorders using the same.
Owner:BRISTOL MYERS SQUIBB CO
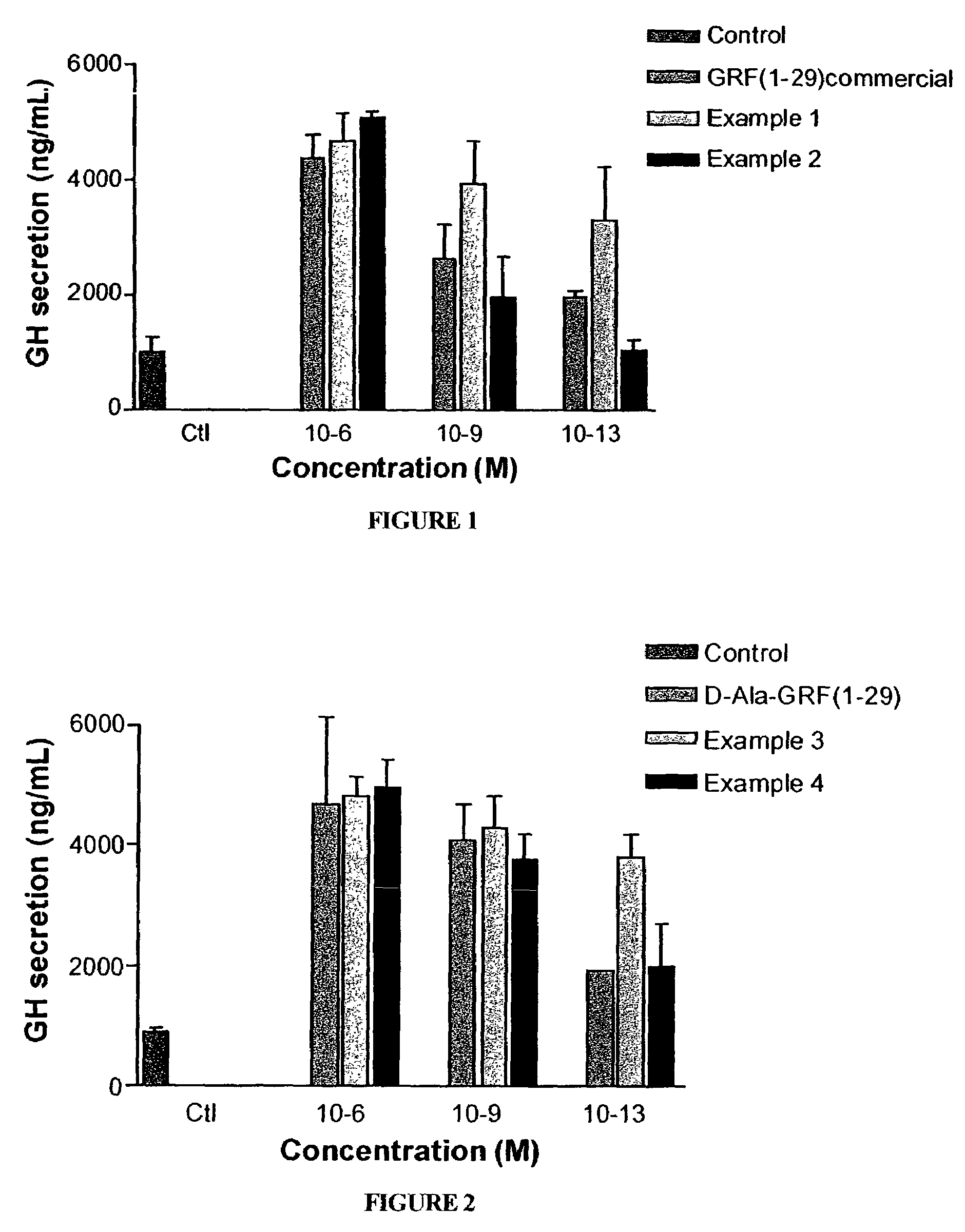
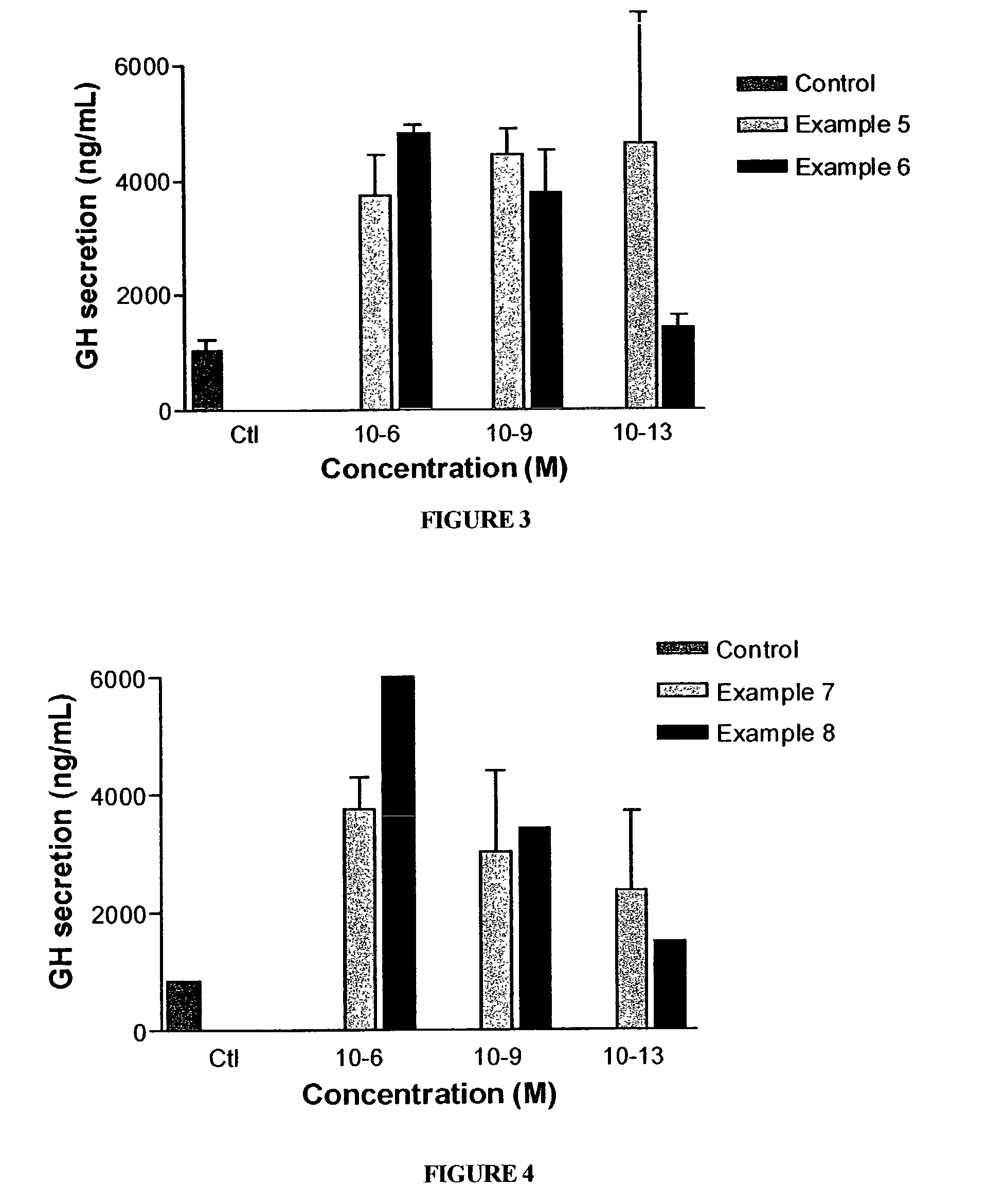
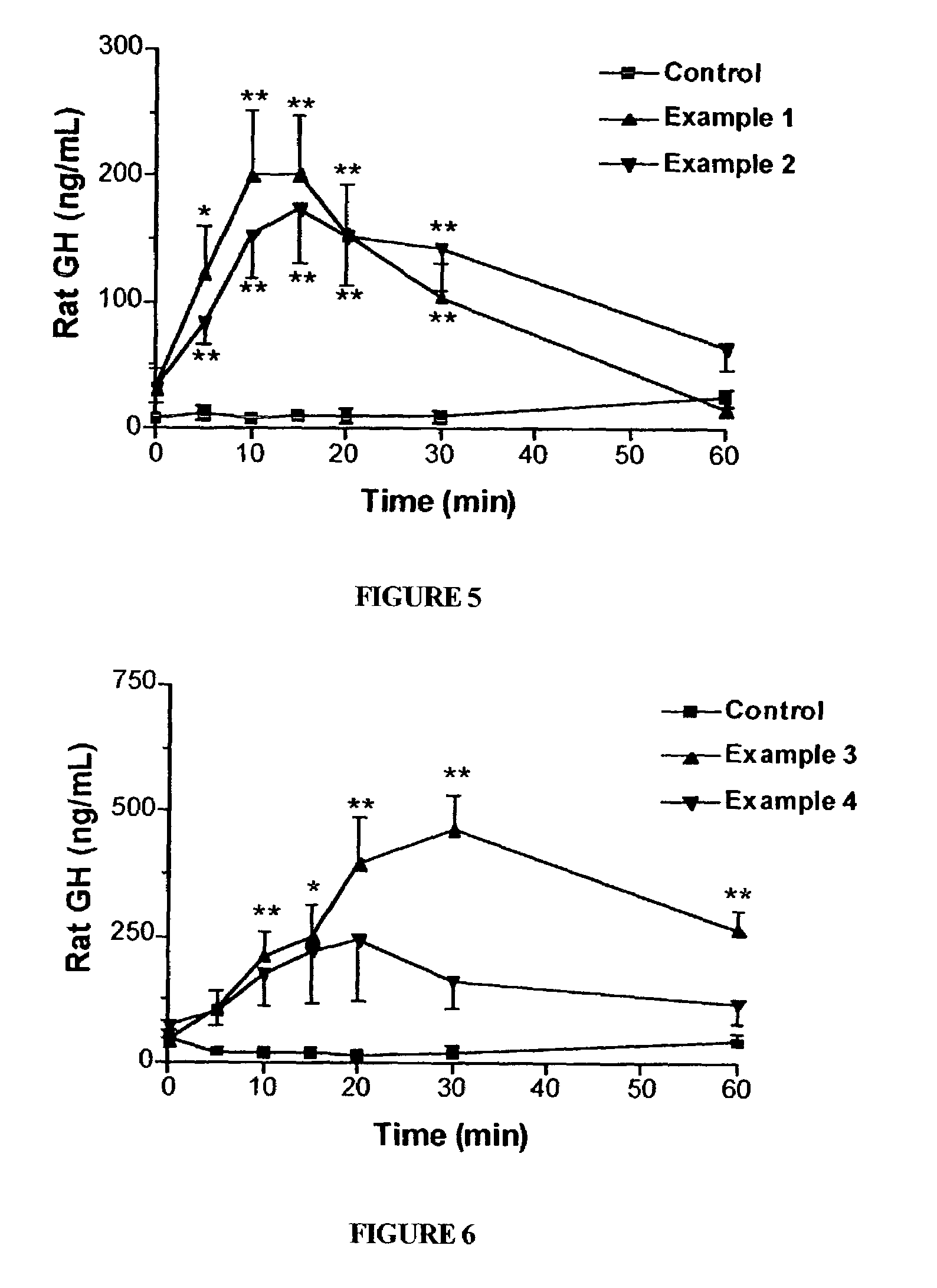
Long lasting growth hormone releasing factor derivatives
InactiveUS7268113B2Prolong half-life in vivoPeptide/protein ingredientsMuscular disorderHalf-lifeIn vivo
This invention relates to growth hormone releasing factor (GRF) derivatives. In particular, this invention relates to GRF peptide derivatives having an extended in vivo half-life, for promoting the endogenous production or release of growth hormone in humans and animals.
Owner:CONJUCHEM
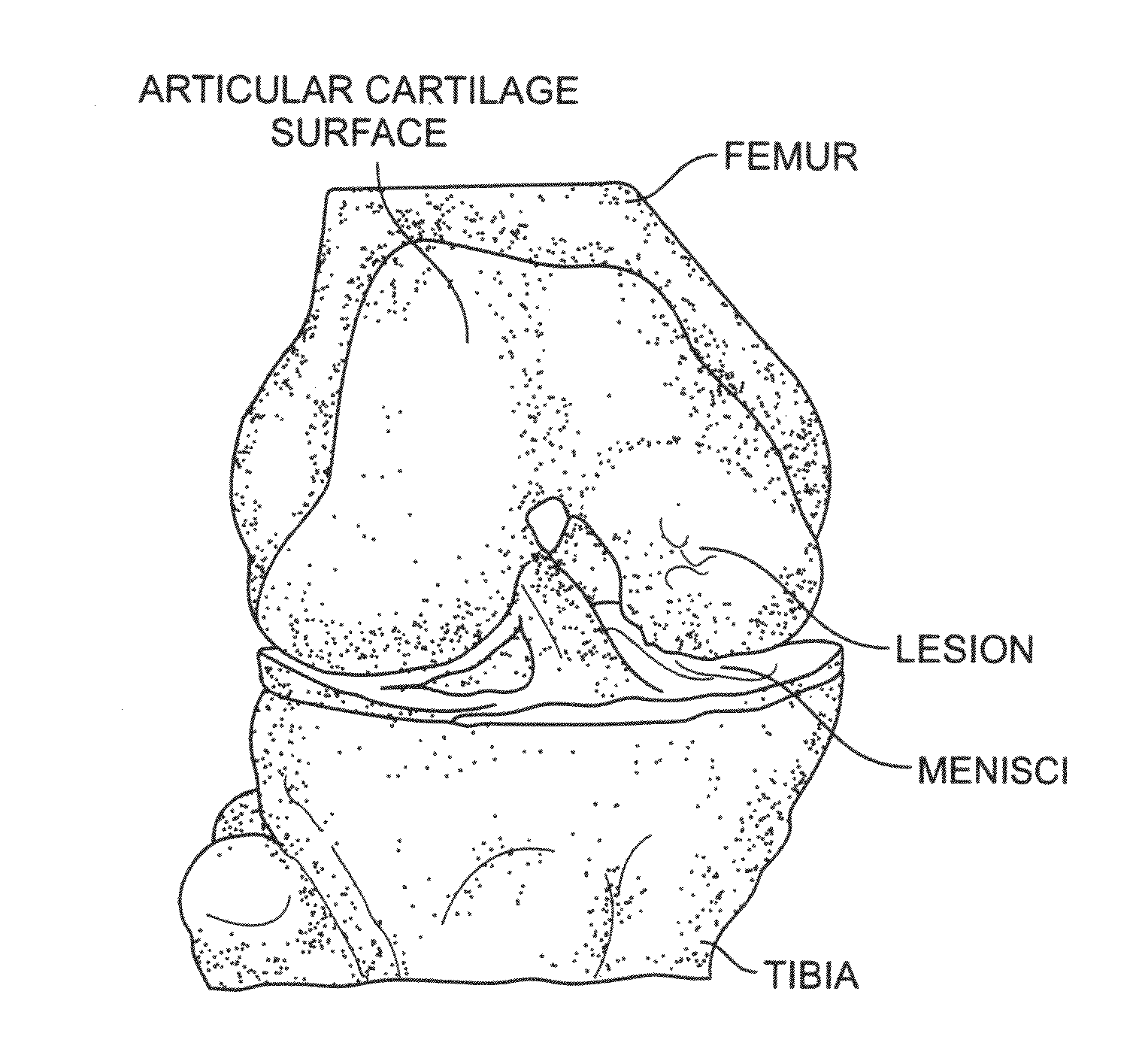
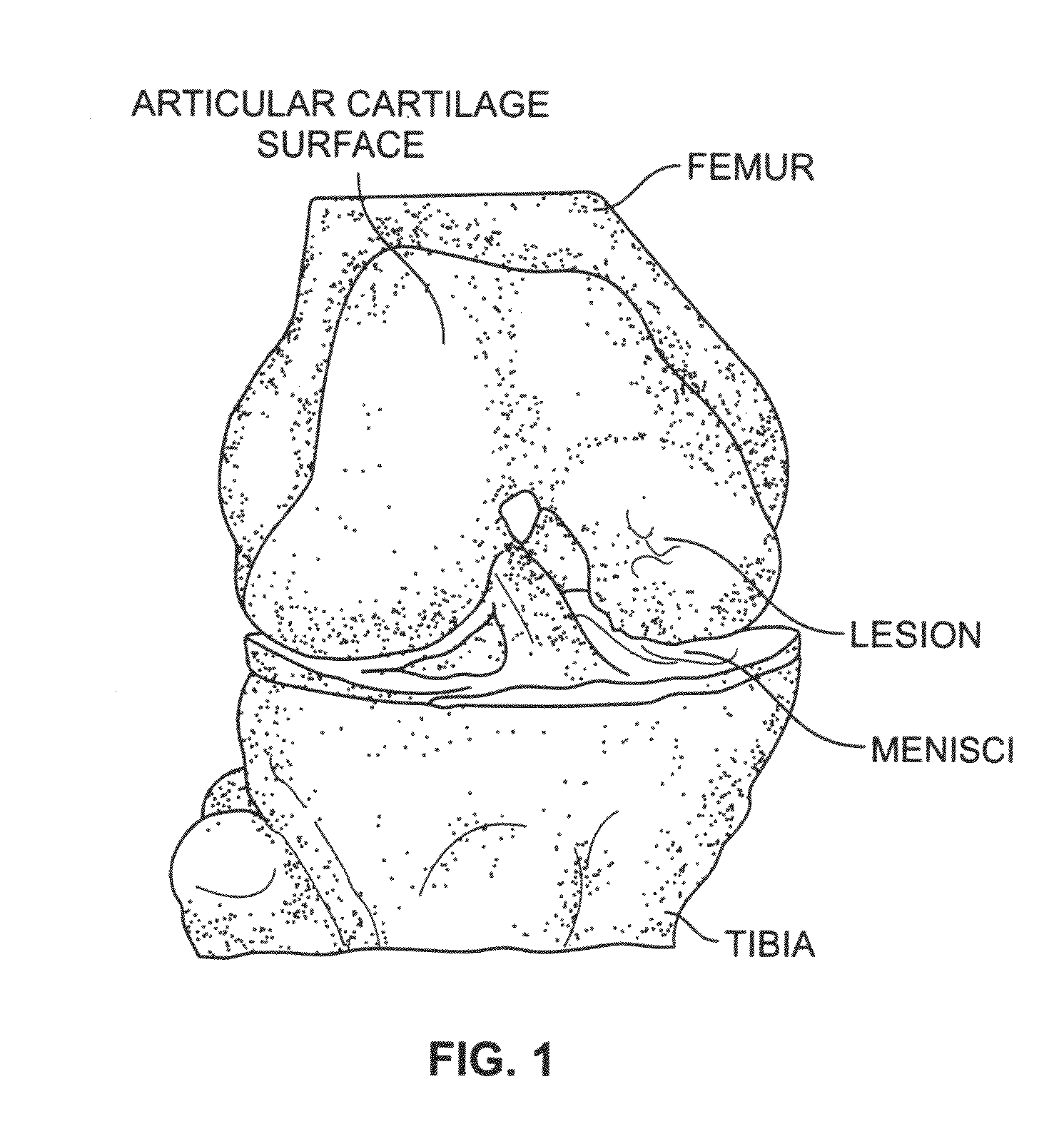
Platelet-derived growth factor compositions and methods for the treatment of osteochondral defects
InactiveUS20100247651A1Increasing cell cell growthIncrease the number of cellsPowder deliveryPeptide/protein ingredientsMedicinePlatelet
The present invention provides compositions and methods for treating an osteochondral defect. In one embodiment, provided is a composition for treating an osteochondral defect comprising a biphasic biocompatible matrix and platelet derived growth factor (PDGF), wherein the biphasic biocompatible matrix comprises a scaffolding material and wherein the scaffolding material forms a porous structure comprising an osseous phase and a cartilage phase. In another embodiment, also provided is a method for treating an osteochondral defect in an individual comprising administering to the individual an effective amount of a composition comprising a biphasic biocompatible matrix and PDGF to at least one site of the osteochondral defect, wherein the biphasic biocompatible matrix comprises a scaffolding material and wherein the scaffolding material forms a porous structure comprising an osseous phase and a cartilage phase.
Owner:BIOMIMETIC THERAPEUTICS INC
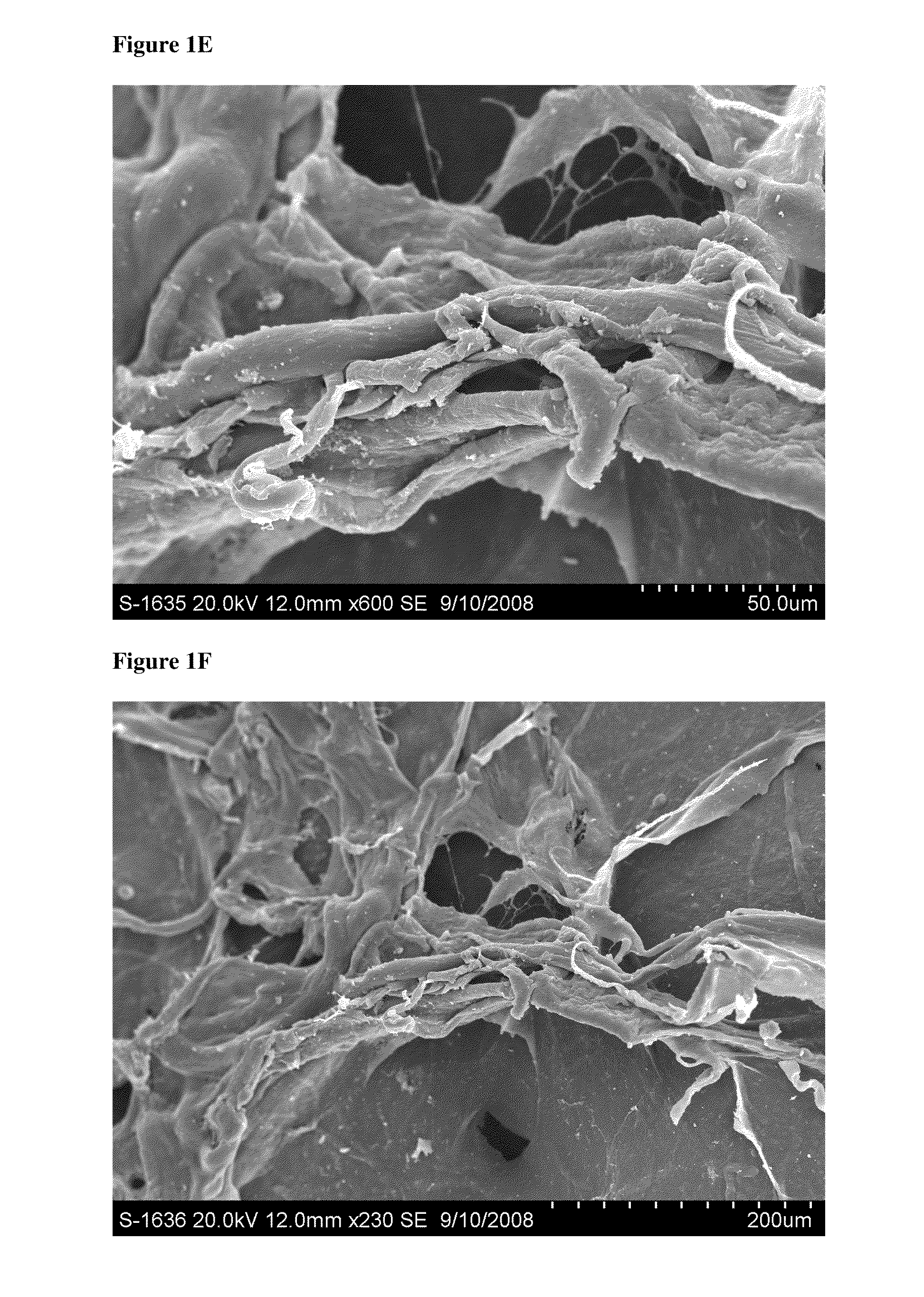
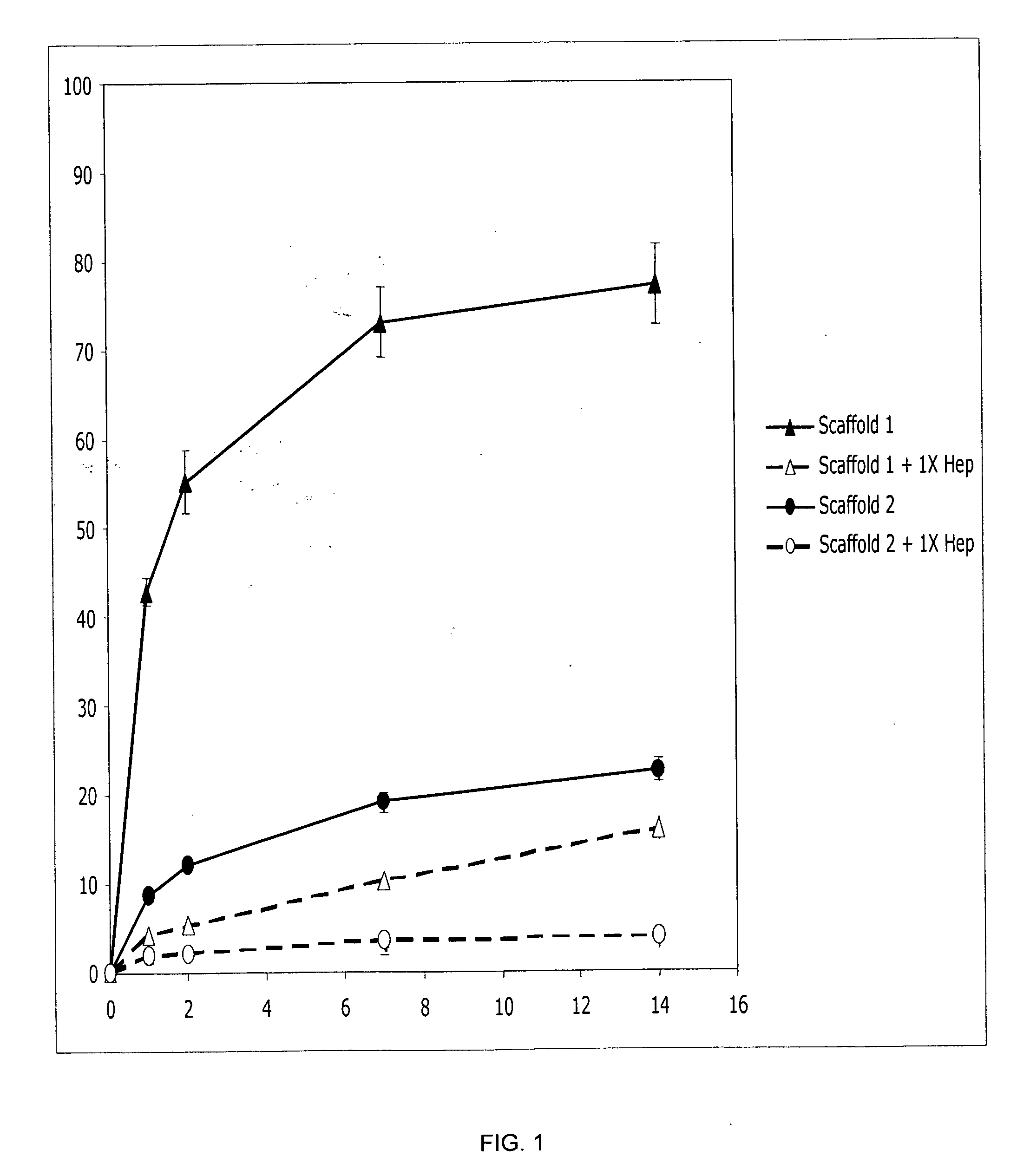
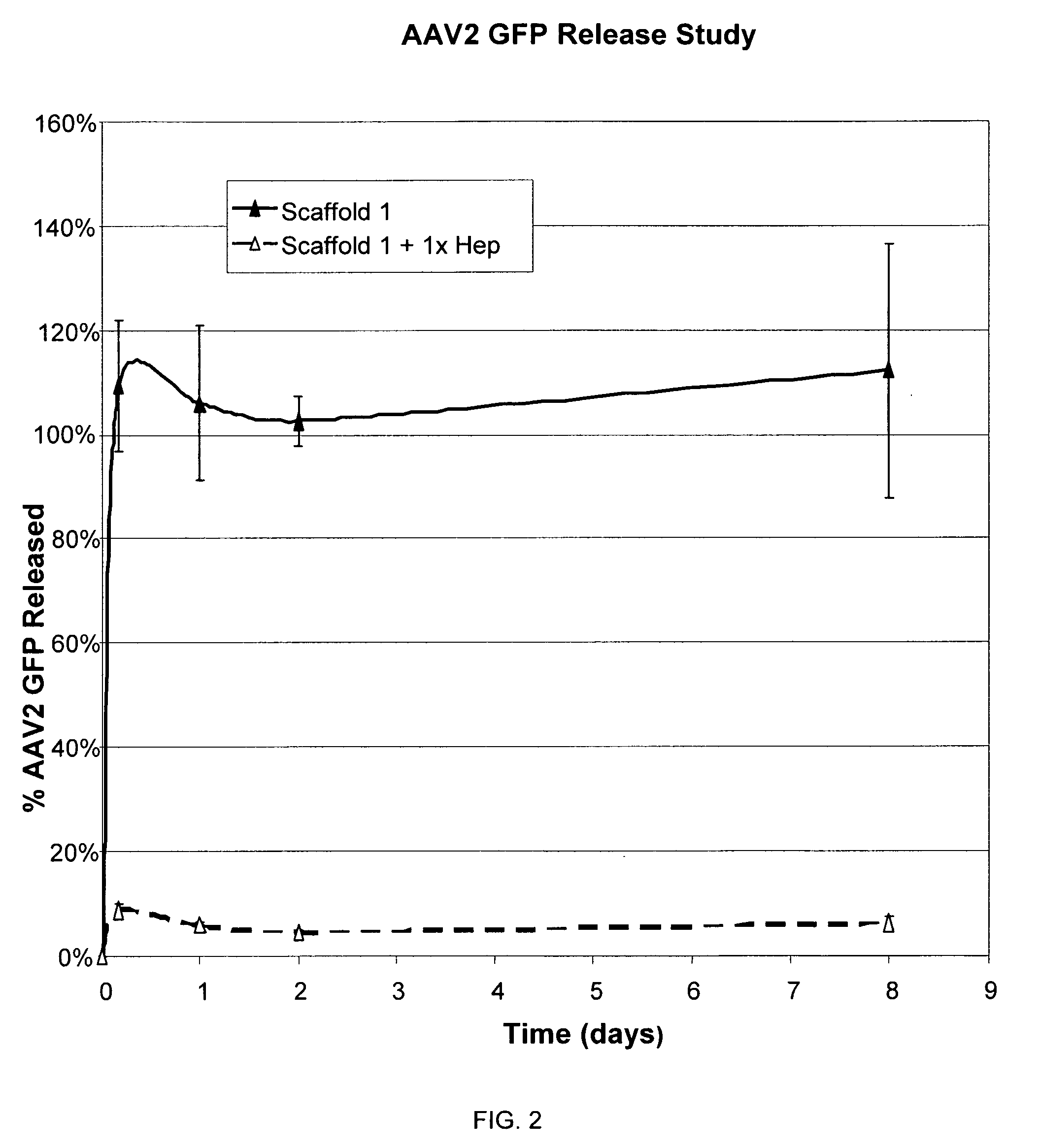
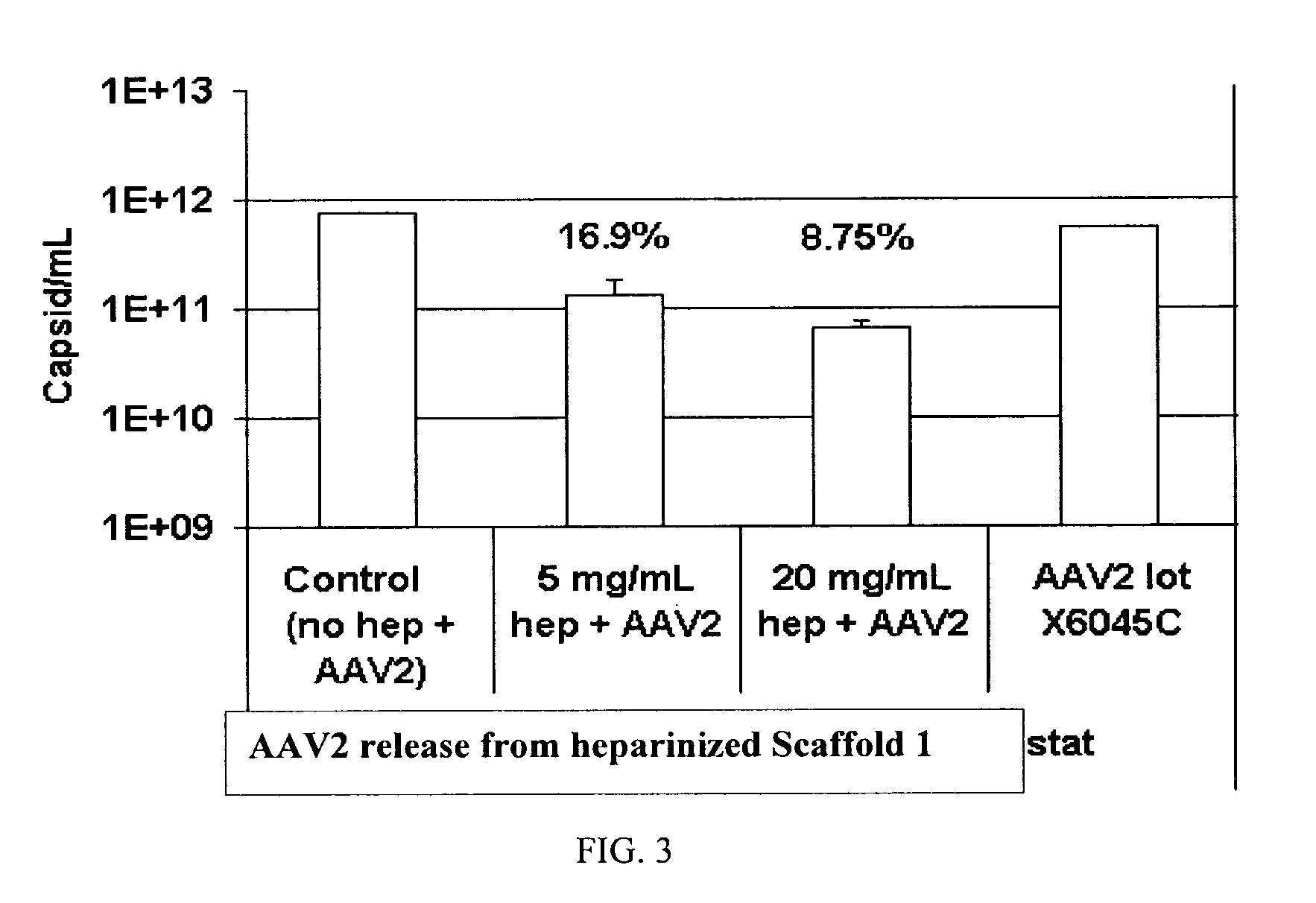
Prolonged delivery of heparin-binding growth factors from heparin-derivatized collagen
InactiveUS20090192079A1Improve biological activityPeptide/protein ingredientsGenetic therapy composition manufactureMuscle tissueCollagen scaffold
The present invention relates to a heparin-derivatized collagen matrix comprising a fragment of heparin covalently linked to a collagen scaffold, wherein the fragment of heparin has molecular weight of less than about 15 kDa, and at least one heparin-binding growth factor (HBGF) or heparin-binding adeno-associated virus (HB-AAV) or a combination thereof and methods for promoting bone growth, bone repair, cartilage repair, bone development, neo-angiogensis, wound healing, tissue engraftment and muscle tissue regeneration and / or tissue augmentation comprising administering a heparin-derivatized collagen matrix that includes at least one heparin-binding growth factor or heparin-binding adeno-associated virus or a combination thereof.
Owner:GENZYME CORP
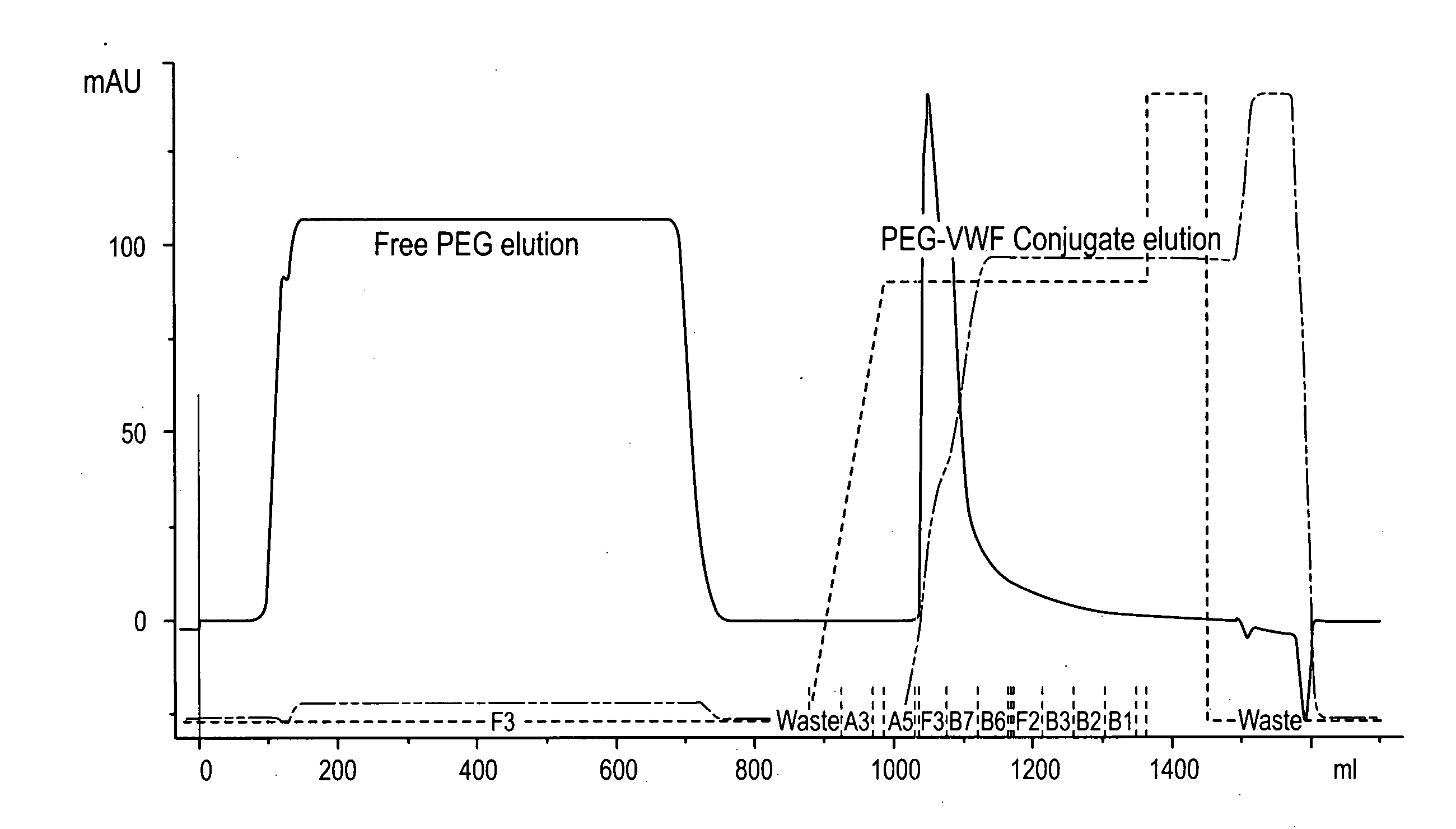
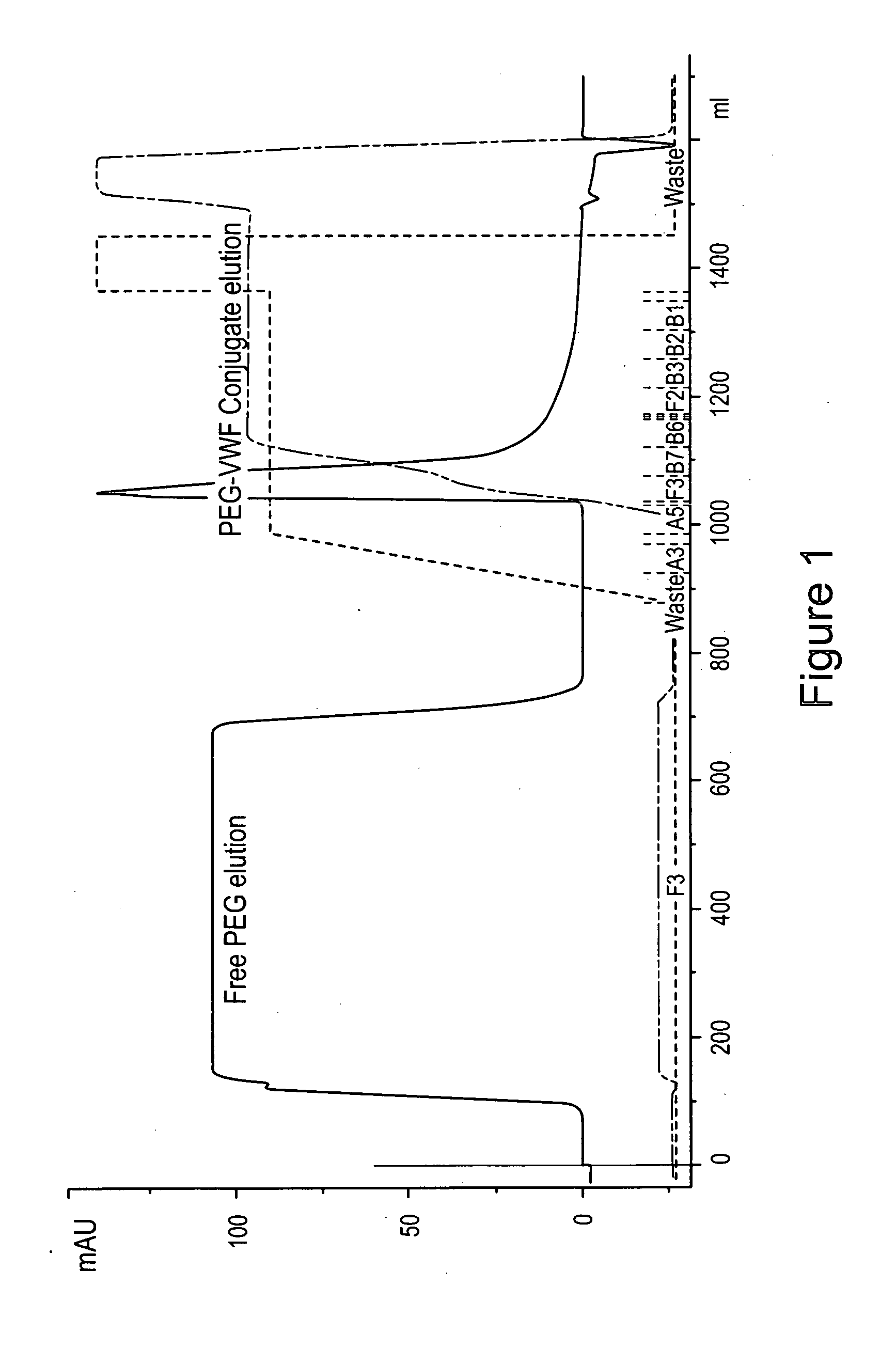
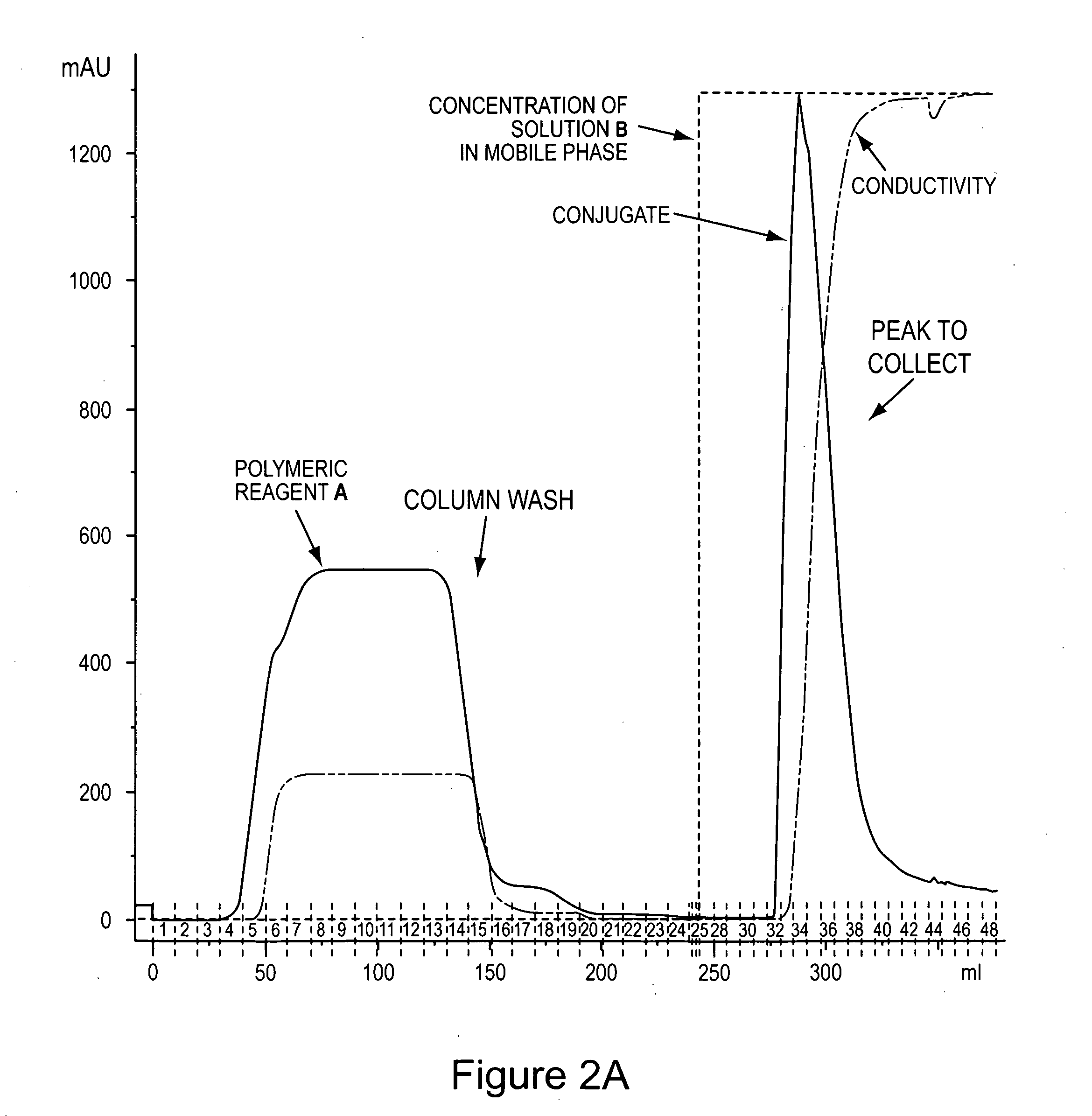
Von Willebrand factor-and factor VIII-polymer conjugates having a releasable linkage
ActiveUS20080234193A1Prolong half-life in vivoFactor VIIPeptide/protein ingredientsFactor VIII vWFFactor ii
The present invention provides von Willebrand Factor-polymer conjugates and Factor VIII-polymer conjugates, each having a releasable linkage. Methods of making conjugates, methods for administering conjugates, are also provided.
Owner:NEKTAR THERAPEUTICS INC +1
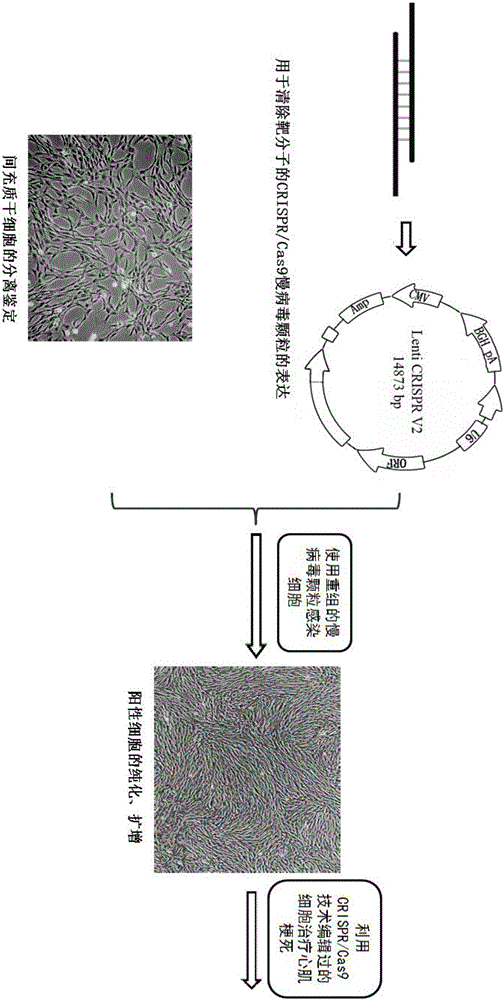
Preparation method of allogenic mesenchymal stem cells by CRISPR (clustered regularly interspaced short palindromic repeats) technique editing and IGF (insulin-like growth factor) optimization and application of allogenic mesenchymal stem cells in treating myocardial infarction
ActiveCN105985985AImprove anti-apoptotic abilityPromote homingUnknown materialsFermentationAntigenInflammatory factors
The invention belongs to the field of allogenic mesenchymal stem cells, and particularly relates to a preparation method of allogenic mesenchymal stem cells by CRISPR (clustered regularly interspaced short palindromic repeats) technique editing and IGF (insulin-like growth factor) optimization and application of the allogenic mesenchymal stem cells in treating myocardial infarction. The preparation method comprises the following steps: carrying out separation by density gradient centrifugation to obtain allogenic single karyocytes, and carrying out adherent culture to obtain mesenchymal stem cells; designing a mesenchymal stem cell surface antigen B2M-gRNA and an inflammatory factor TNF-alpha-gRNA; establishing recombinant slow virus particles, and transfecting the mesenchymal stem cells; optimizing the mesenchymal stem cells by using IGF-1; and preparing drugs for treating myocardial infarctions by using the modified and optimized mesenchymal stem cells. The CRISPR / Cas9 technique is utilized to remove the antigens capable of causing immunological rejection and the inflammatory factors capable of causing inflammatory reaction on the mesenchymal stem cell surface, and the IGF-1 is utilized to enhance the apoptosis resistance of the mesenchymal stem cells and promote the homing of the mesenchymal stem cells, thereby providing a new technical scheme for preparing drugs for treating cardiovascular diseases in clinic. The prepared allogenic mesenchymal stem cells can not cause immunological rejection after cell transplantation.
Owner:SUZHOU UNIV
Heterocyclic compounds regulating clotting
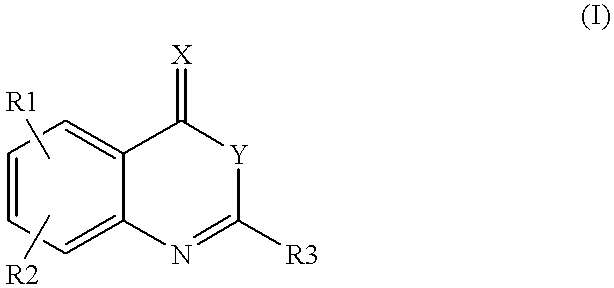
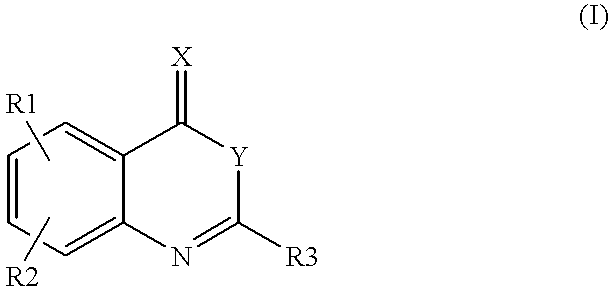
InactiveUS6180625B1Inhibition formationPreventing initiationOrganic active ingredientsOrganic chemistryFactor VIIaFactor VII
Compounds of formula (I)as factor VII-tissue factor inhibitors as well as novel benzoxazin derivatives are disclosed, wherein R1, R2, R3, X and Y are as defined in the specification. These compounds, and pharmaceutically acceptable salts thereof, have been shown to be inhibitors of factor VIIa-tissue factor activity and have anticoagulant properties. These compounds are useful for treating deficiencies of blood clotting factors or the effects of inhibitors to blood clotting factors. Methods for inhibiting clotting activity are also disclosed.
Owner:NOVO NORDISK AS
Synthetic heparin-binding growth factor analogs
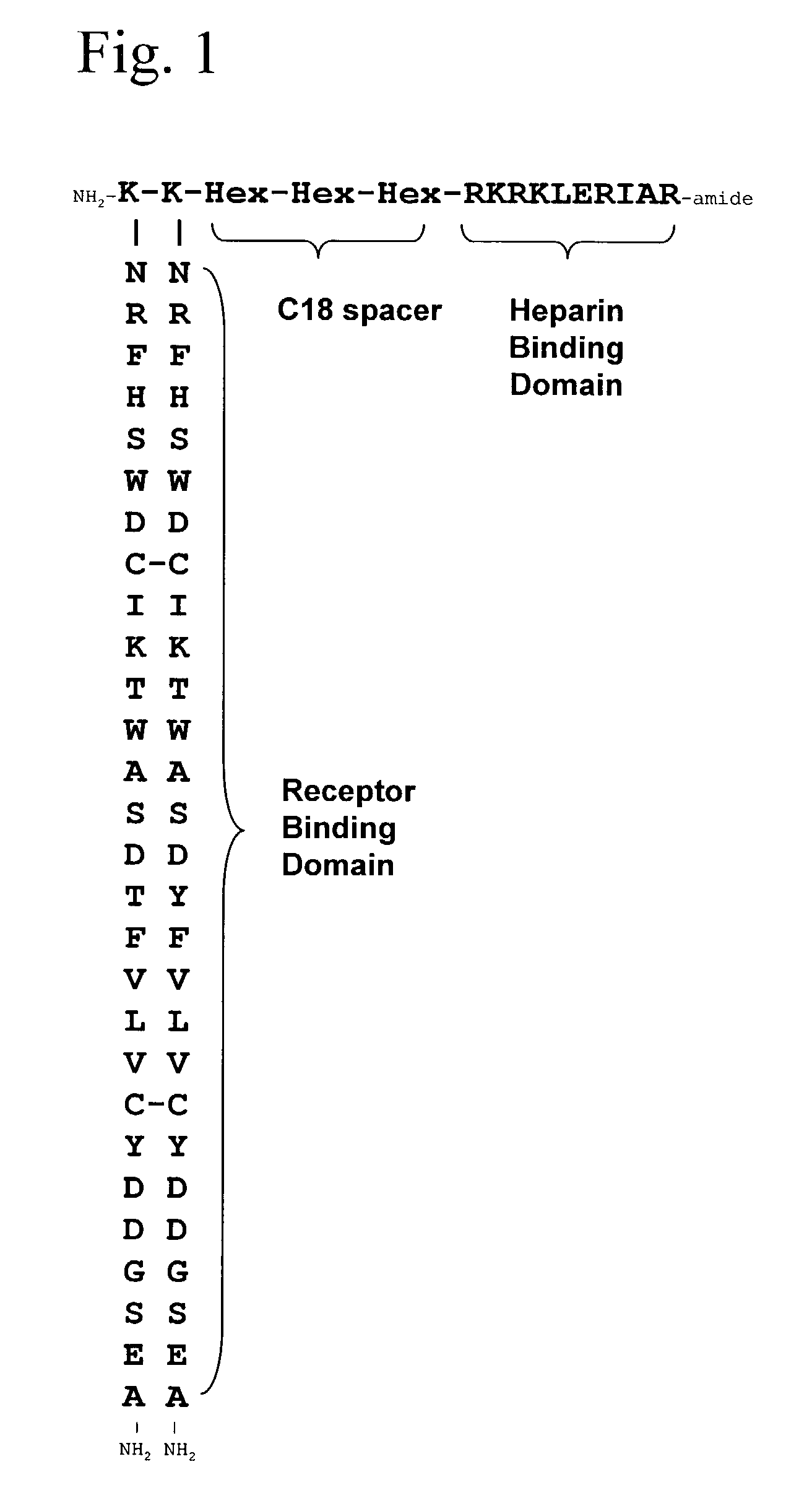
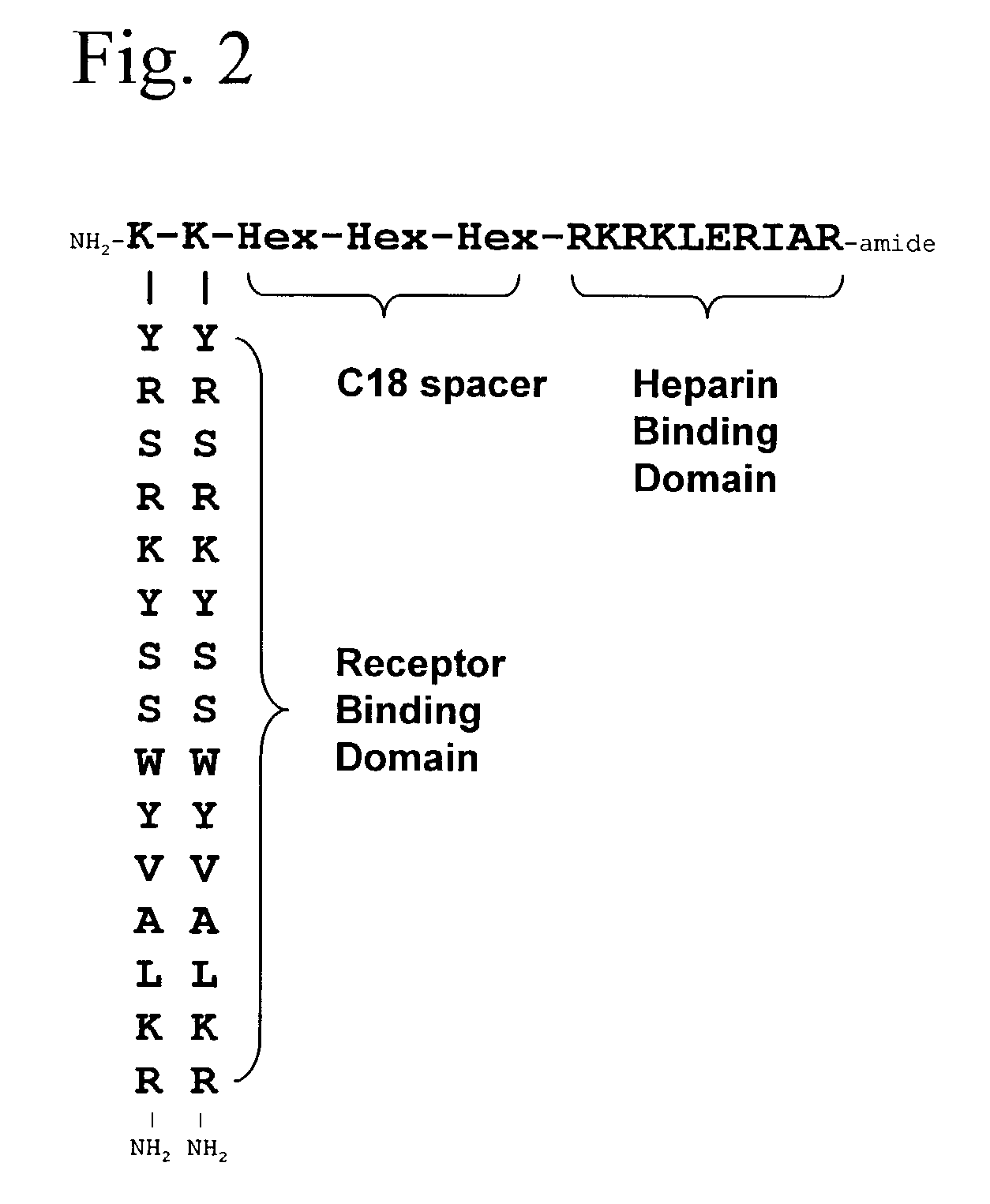
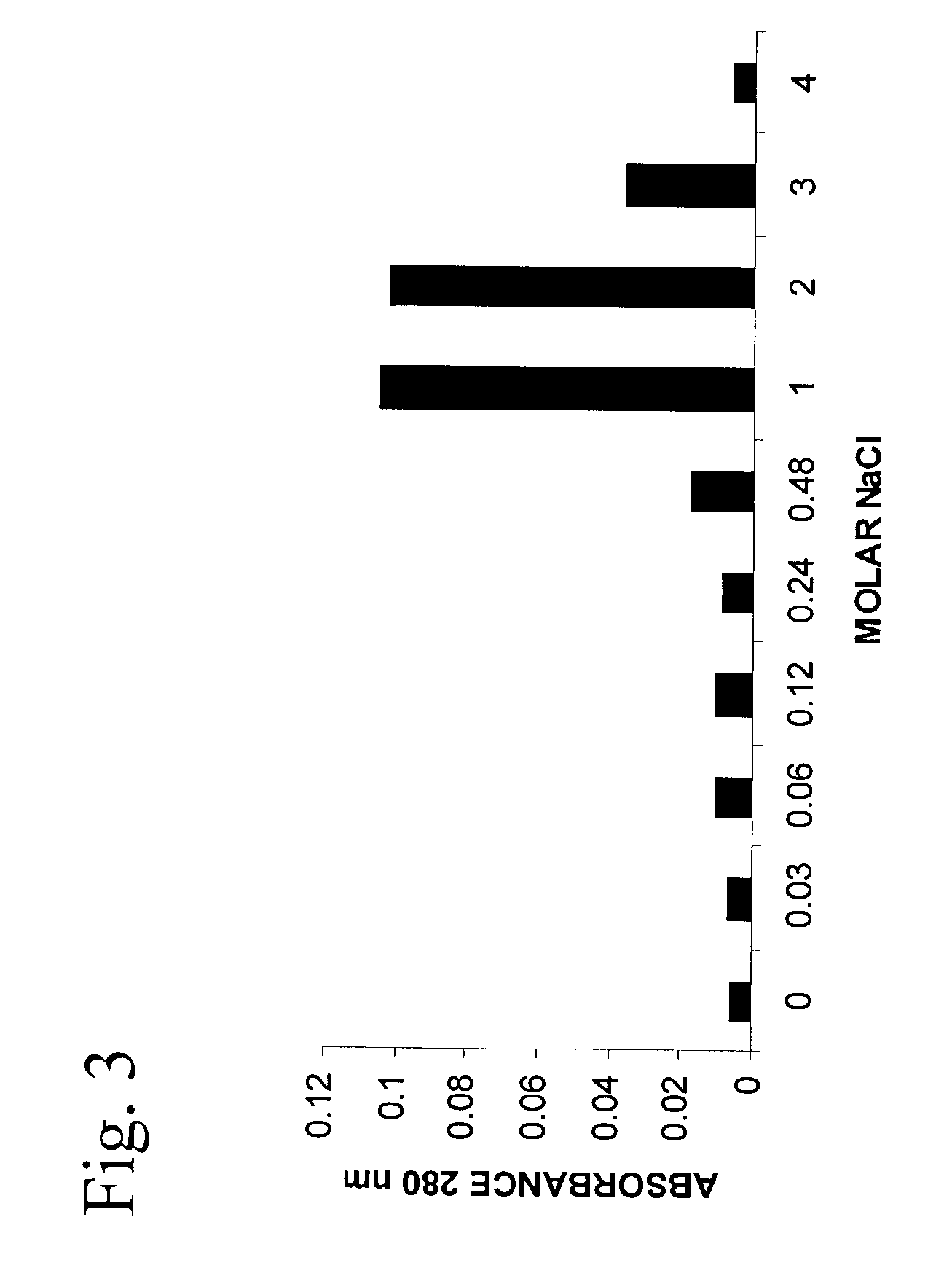
InactiveUS7166574B2Ameliorate harmful effect of radiationPeptide/protein ingredientsAntibody mimetics/scaffoldsFactor iiBinding domain
The invention provides synthetic heparin-binding growth factor analogs having at least one peptide chain that binds a heparin-binding growth factor receptor, covalently bound to a hydrophobic linker, which is in turn covalently bound to a non-signaling peptide that includes a heparin-binding domain. The synthetic heparin-binding growth factor analogs are useful as soluble biologics or as surface coatings for medical devices.
Owner:BROOKHAVEN SCI ASSOCS +1
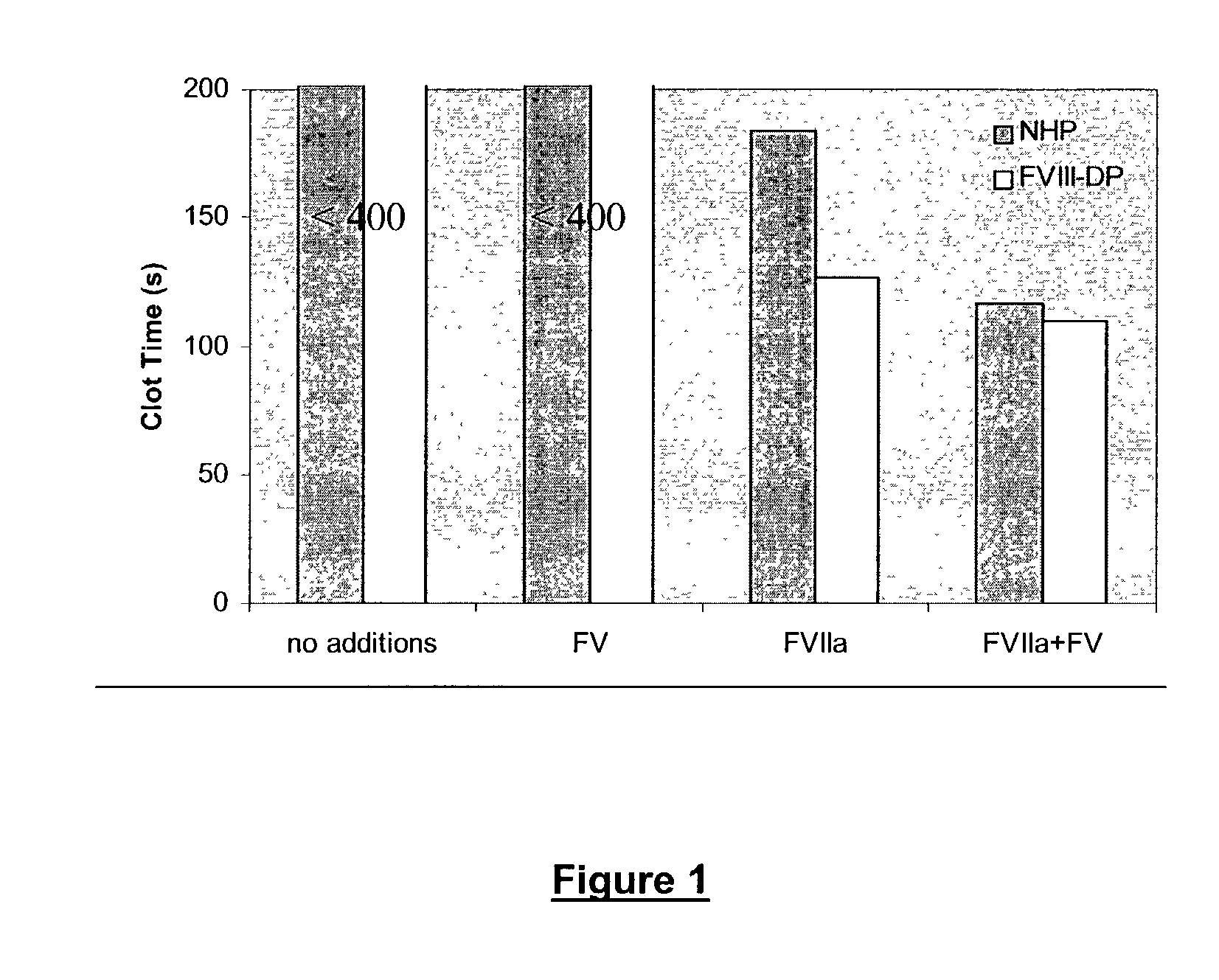
Pharmaceutical composition comprising factor VII polypeptides and factor V polypeptides
InactiveUS7125846B2Improved and reliable and widely applicableGood coagulationPeptide/protein ingredientsMammal material medical ingredientsFactor iiBleeding episodes
The present invention relates to a composition comprising factor VII or a factor VII-related polypeptide, and factor V or a factor V-related polypeptide, and the use thereof for treating bleeding episodes.
Owner:NOVO NORDISK AS
Cartilage particle tissue mixtures optionally combined with a cancellous construct
Mixtures, such as gels or pastes, comprising freeze-milled cartilage particles and exogenous growth factors are used for repairing chondral defects. Such mixtures may be applied to constructs comprising cancellous bone for implantation at the defect site. Suitable growth factors include variants of FGF-2, particularly variants that include a sole amino acid substitution for asparagine at amino acid 111 of the β8-β9 loop of the FGF-2 peptide. Such FGF-2 variants are released slowly and continuously at a constant rate from cartilage pastes. In other embodiments, the amino acid substituted for asparigine is glycine. Other variants that may be used include FGF-9 variants having truncated chains and a sole amino acid substitution in the β8-β9 loop of the FGF-9 peptide either for tryptophan at amino acid 144 or for asparagine at amino acid 143.
Owner:MUSCULOSKELETAL TRANSPLANT FOUND INC +1
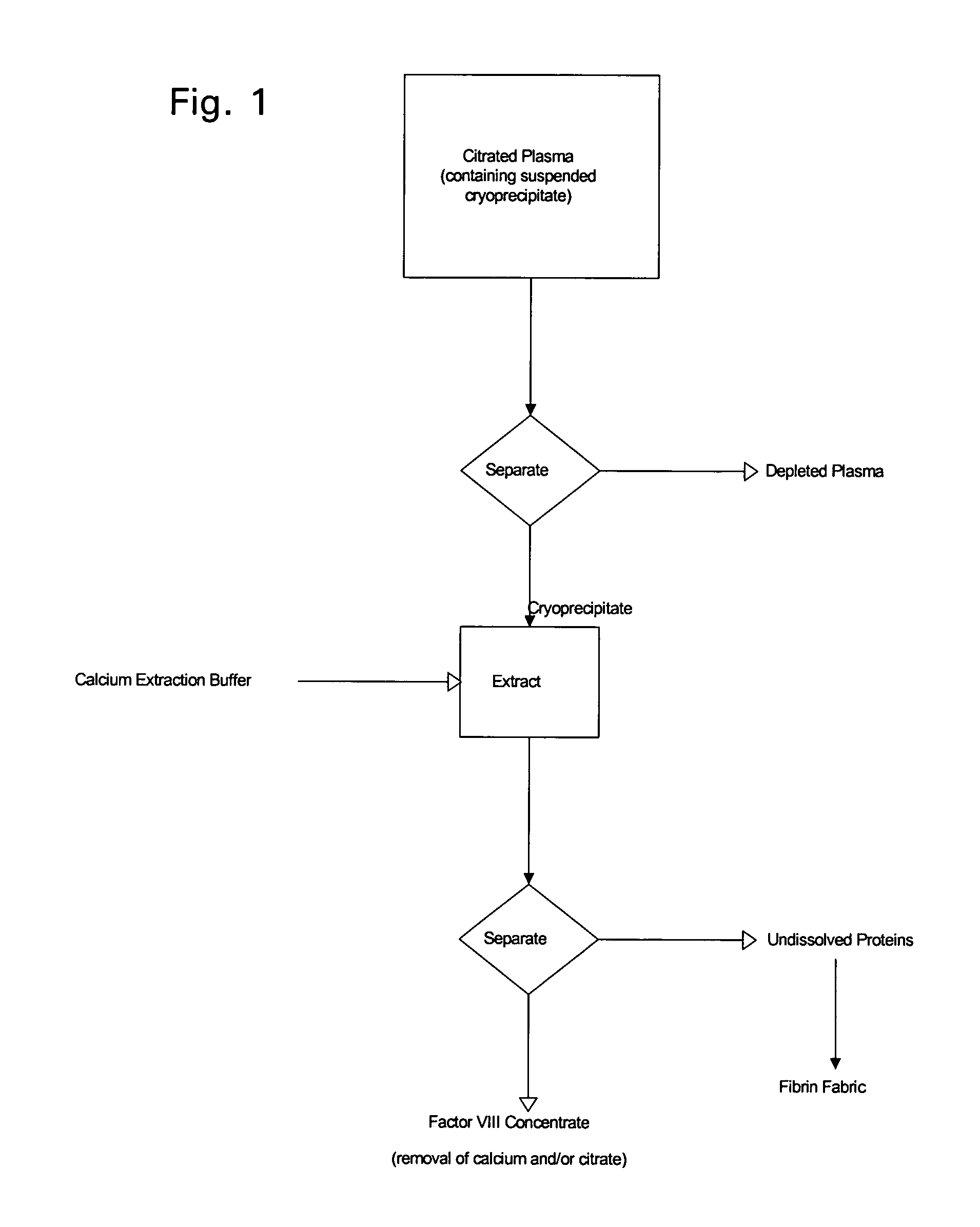
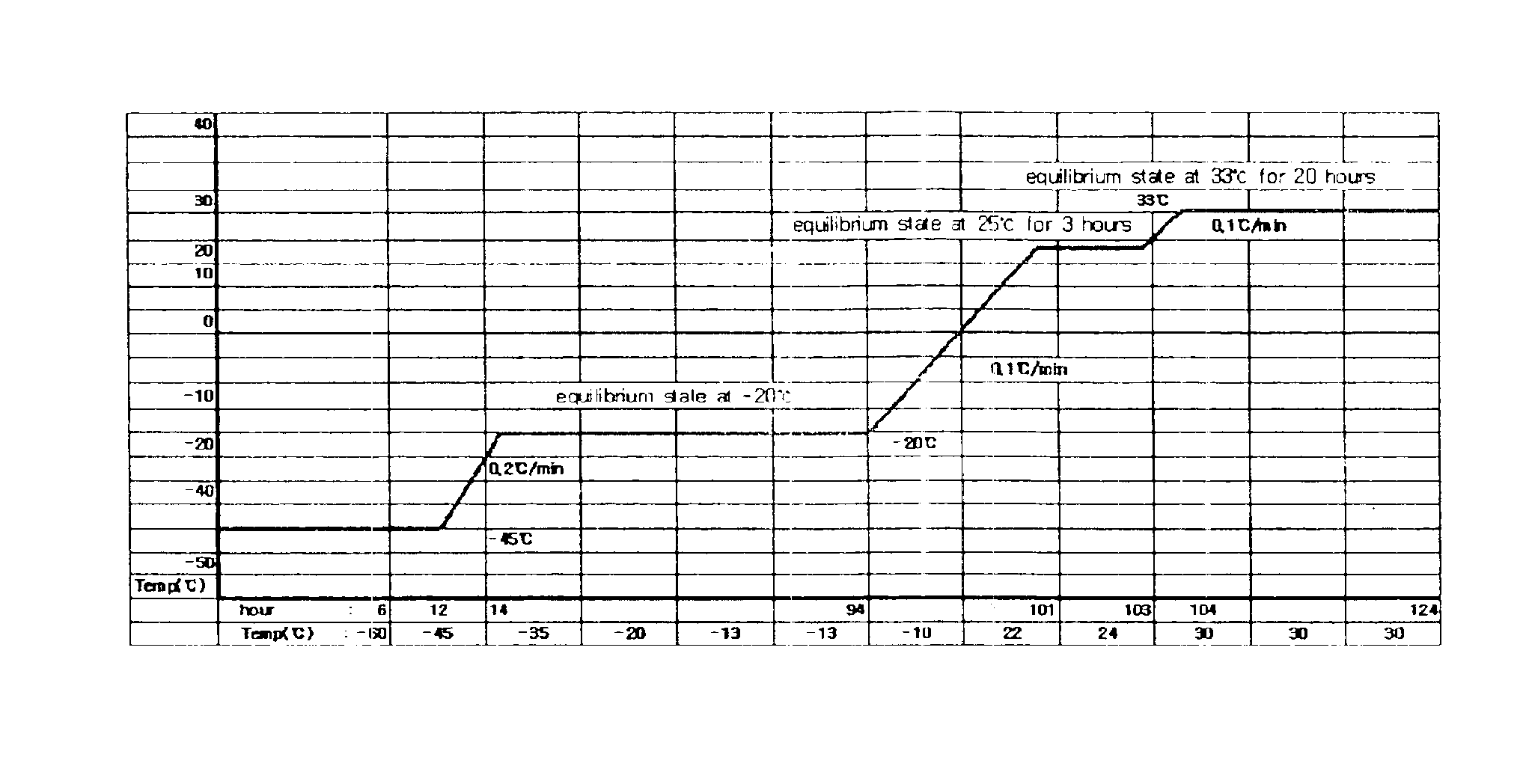
Enhanced production of blood clotting factors and fibrin fabric
The blood collection, processing and transfer by separation of discrete components containing additional citrate (at least about trisodium citrate 9% w / v) in one or other of collection or processing bag provides for enhanced yield and purity of cryoprecipitate. Inhibiting the activation or denaturation of blood components including blood cells and plasma proteins and with the removal of the activated and denatured components thereby improving safety and efficacy of end products. The inventive process is particularly suited to an improved extraction process to yield concentrated clotting factors from single donors or limited pools without use of chromatography. Following extraction the remaining cryoprecipitate can advantageously be formed into a fibrin fabric used in surgeries and in the treatment of wounds.
Owner:SHANBROM TECH
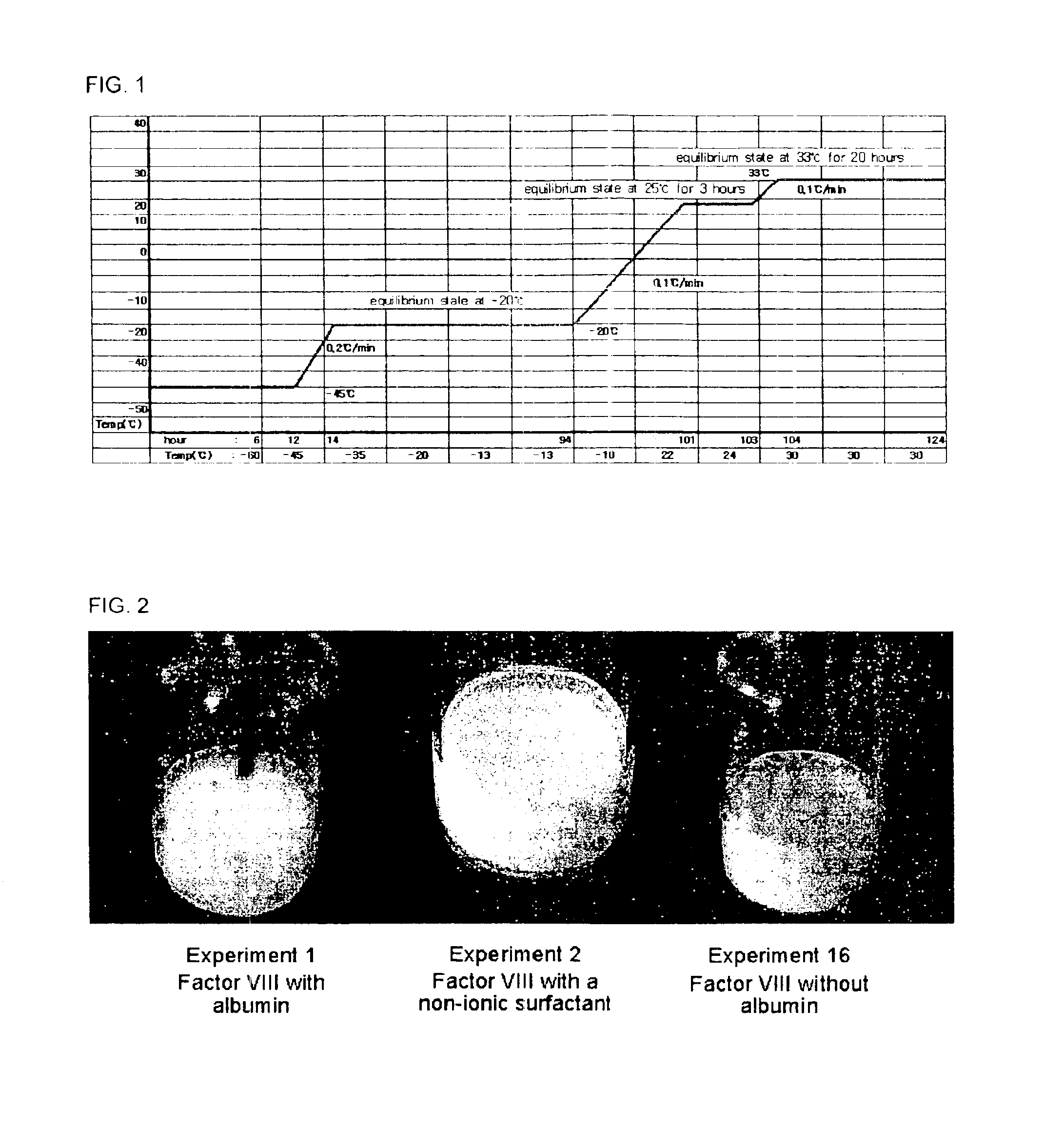
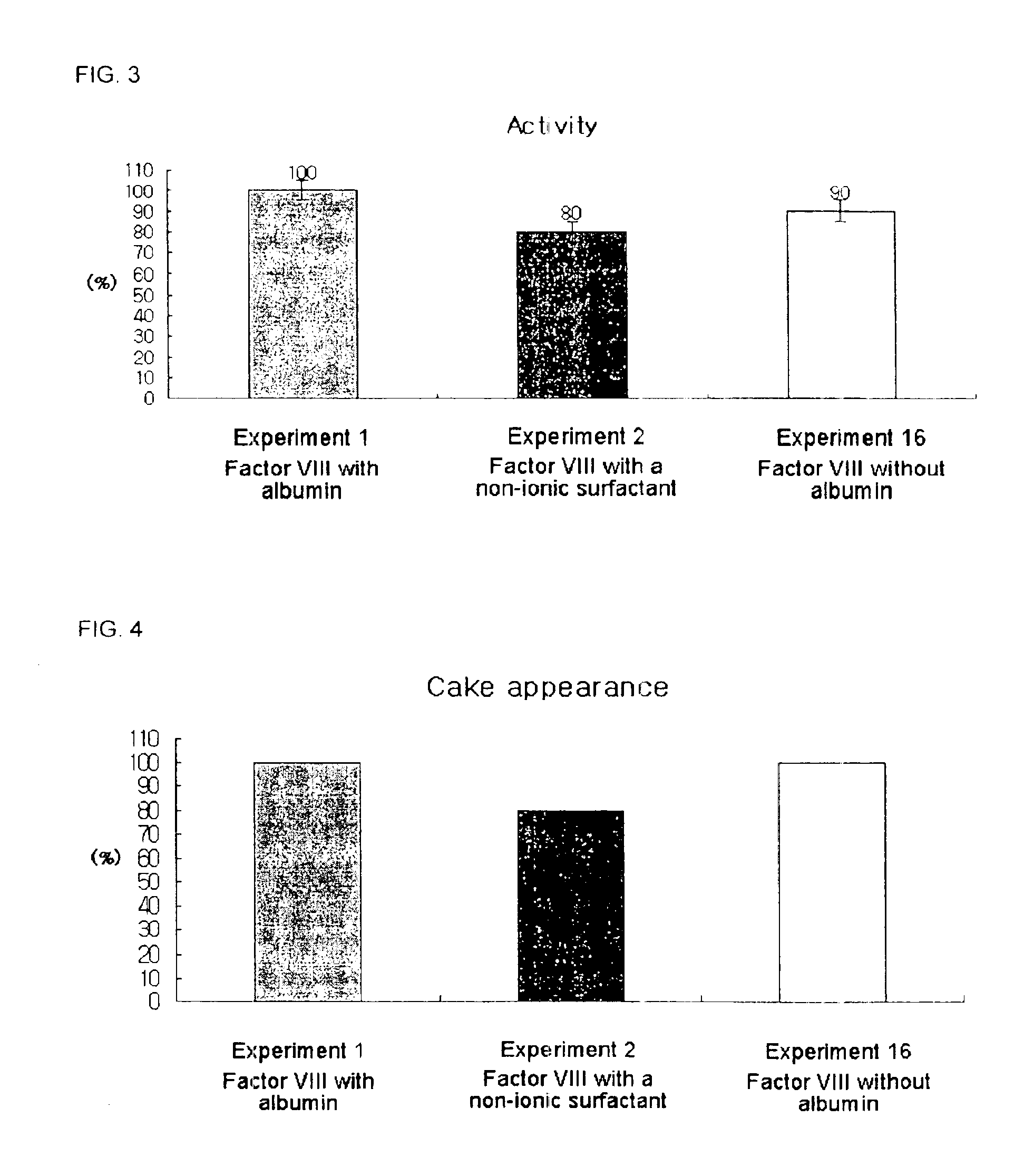
Pharmaceutical preparation of recombinant factor VIII lyophilized without albumin as a stabilizer
ActiveUS6887852B1Same pharmaceutical efficacyAvoid virus infectionFactor VIIPowder deliveryMedicineArginine
Disclosed is a lyophilized preparation of recombinant factor VIII used as a therapeutic preparation of hemophilia A. The lyophilized preparation of recombinant factor VIII is prepared by performing lyophilization using a mixture comprising 6 to 100 mM of L-arginine, 3.5 to 50 mM of L-isoleucine, and 10 to 100 mM of L-glutamic acid as a stabilizer for stabilizing the recombinant factor VIII which exhibits an unstable activity during lyophilization, rather than using human blood derived albumin.
Owner:KOREA GREEN CROSS CORP
Antibody Substituting for Function of Blood Coagulation Factor VIII
InactiveUS20120237517A1Shorten clotting timeImmunoglobulins against blood coagulation factorsAntibody ingredientsBlood coagulation factor VIIIBispecific antibody
The present inventors produced a variety of bispecific antibodies that specifically bind to both F. IX / F. IXa and F. X, and functionally substitute for F. VIIIa, i.e., have a cofactor function to promote F. X activation via F. IXa. Among these antibodies, the antibody A44 / B26 reduced coagulation time by 50 seconds or more as compared to that observed when the antibody was not added. The present inventors produced a commonly shared L chain antibody from this antibody using L chains of A44, and showed that A44L can be used as commonly shared L chains, although the activity of the resulting antibody is reduced compared to the original antibody (A44HL-B26HL). Further, with appropriate CDR shuffling, the present inventors successfully produced highly active multispecific antibodies that functionally substitute for coagulation factor VIII.
Owner:CHUGAI PHARMA CO LTD
Use of heparinase to decrease inflammatory responses
InactiveUS20010006635A1Inhibiting leukocyte rollingInhibiting chemokine gradient formationOrganic active ingredientsPowder deliveryWhite blood cellDigestion
Heparinase enzymes can be used as a medical treatment to reduce localized inflammatory responses. Treatment of activated endothelium with heparinase inhibits leukocyte rolling, adhesion and extravasation. Most of the heparin and heparan sulfate on endothelial cell surfaces and in basement membranes is degraded by exposure to heparinase. In addition, immobilized chemokines, which are attached to heparin / heparan sulfate on activated endothelium are solubilized by heparinase digestion. Heparinase can be infused into the vascular system to inhibit accumulation of leukocytes in inflamed tissue and decrease damage resulting from localized inflammations. Targeting of heparinase to activated endothelium can be accomplished through localized administration and / or use of genetically engineered heparinase containing endothelium ligand-binding domains.
Owner:BIOMARIN PHARMA INC +1
Bone Graft
ActiveUS20080145392A1Good curative effectSimple compositionAdditive manufacturing apparatusBone implantOSTEOINDUCTIVE FACTORIn vivo
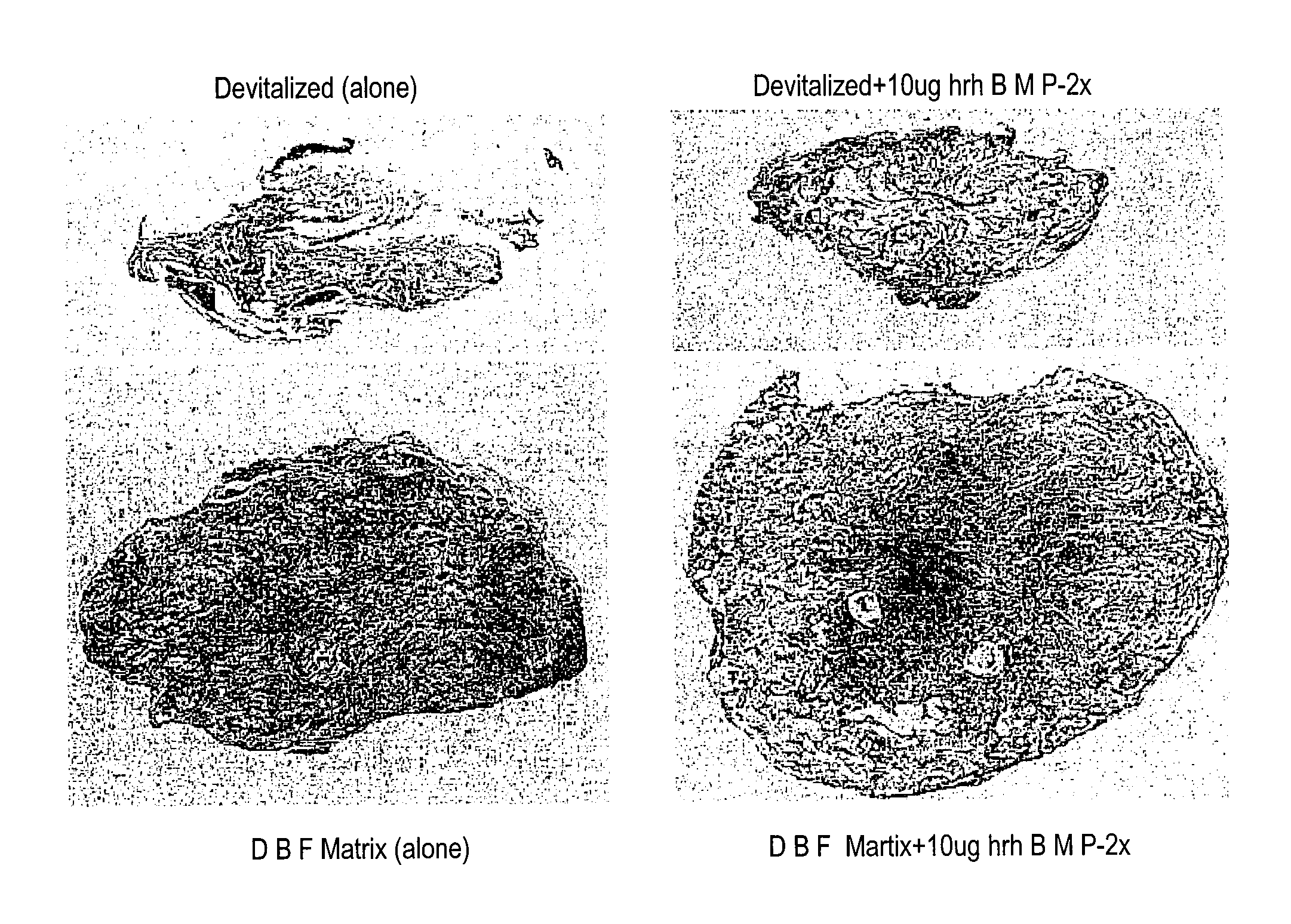
An improved demineralized bone matrix (DBM) or other matrix composition is provided that has been mixed with a stabilizing agent that acts as (1) a diffusion barrier, (2) a enzyme inhibitor, (3) a competitive substrate, or (4) a masking moiety. A diffusion barrier acts as a barrier so as to protect the osteoinductive factors found in DBM from being degraded by proteolytic and glycolytic enzymes at the implantation site. Stabilizing agents may be any biodegradable material such as starches, modified starches, cellulose, dextran, polymers, proteins, and collagen. As the stabilizing agents degrades or dissolves in vivo, the osteoinductive factors such as TGF-.beta., BMP, and IGF are activated or exposed, and the activated factors work to recruit cells from the preivascular space to the site of injury and to cause differentiation into bone-forming cells. The invention also provides methods of preparing, testing, and using the inventive improved osteodinductive matrix compositions
Owner:WARSAW ORTHOPEDIC INC
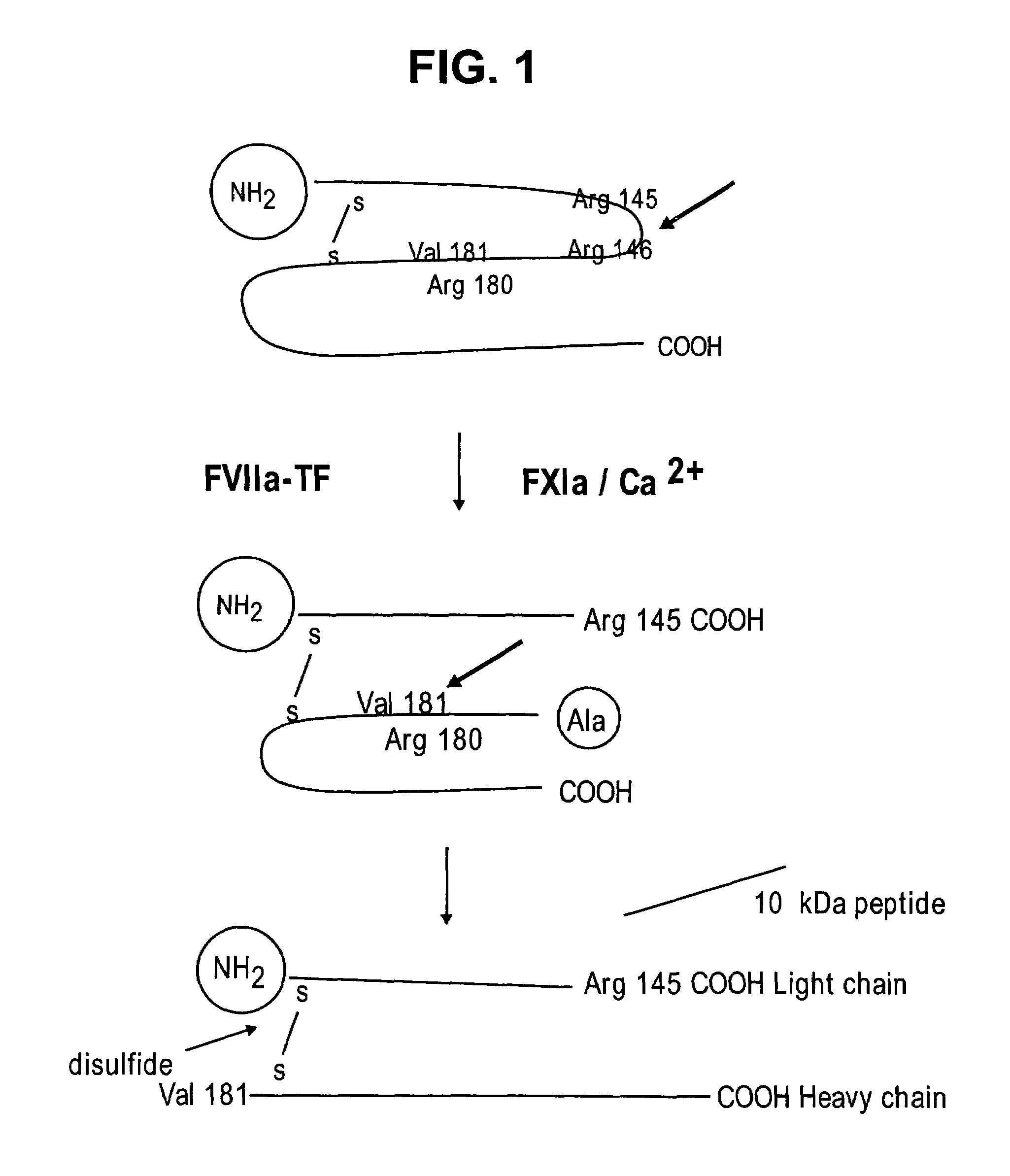
Region of factor IXa protease domain that interacts with factor VIIIa and methods therefor
Novel polypeptides or derivatives comprising the factor VIIIa binding site on factor IXa are disclosed. The novel polypeptides or derivatives have anti-coagulation activity. Nucleic acids encoding those polypeptides are also disclosed. Methods for identifying an agent having anti-coagulation activity are also disclosed. These methods comprise determining whether the agent displaces the polypeptide or derivative from its factor VIIa binding site. The agent identified in these methods is also useful in methods for treating a patient to prevent thrombosis. The treatment methods comprise administration of the agent to the patient. Additional methods are also disclosed for treating a patient to prevent thrombosis, comprising treating the patient with a polypeptide or derivative comprising the factor VIIIa binding site on factor IXa. Methods of preventing coagulation in a blood sample are also disclosed, comprising adding the polypeptides or derivatives described above to the blood sample. Methods of detecting factor VIIIa in a sample are also disclosed. Those methods comprise contacting the sample with the above-described polypeptide or derivative, wherein the polypeptide or derivative also comprises a covalently attached detectable moiety, then determining whether the polypeptide or derivative is binding factor VIIIa from the sample.
Owner:SAINT LOUIS UNIVERSITY
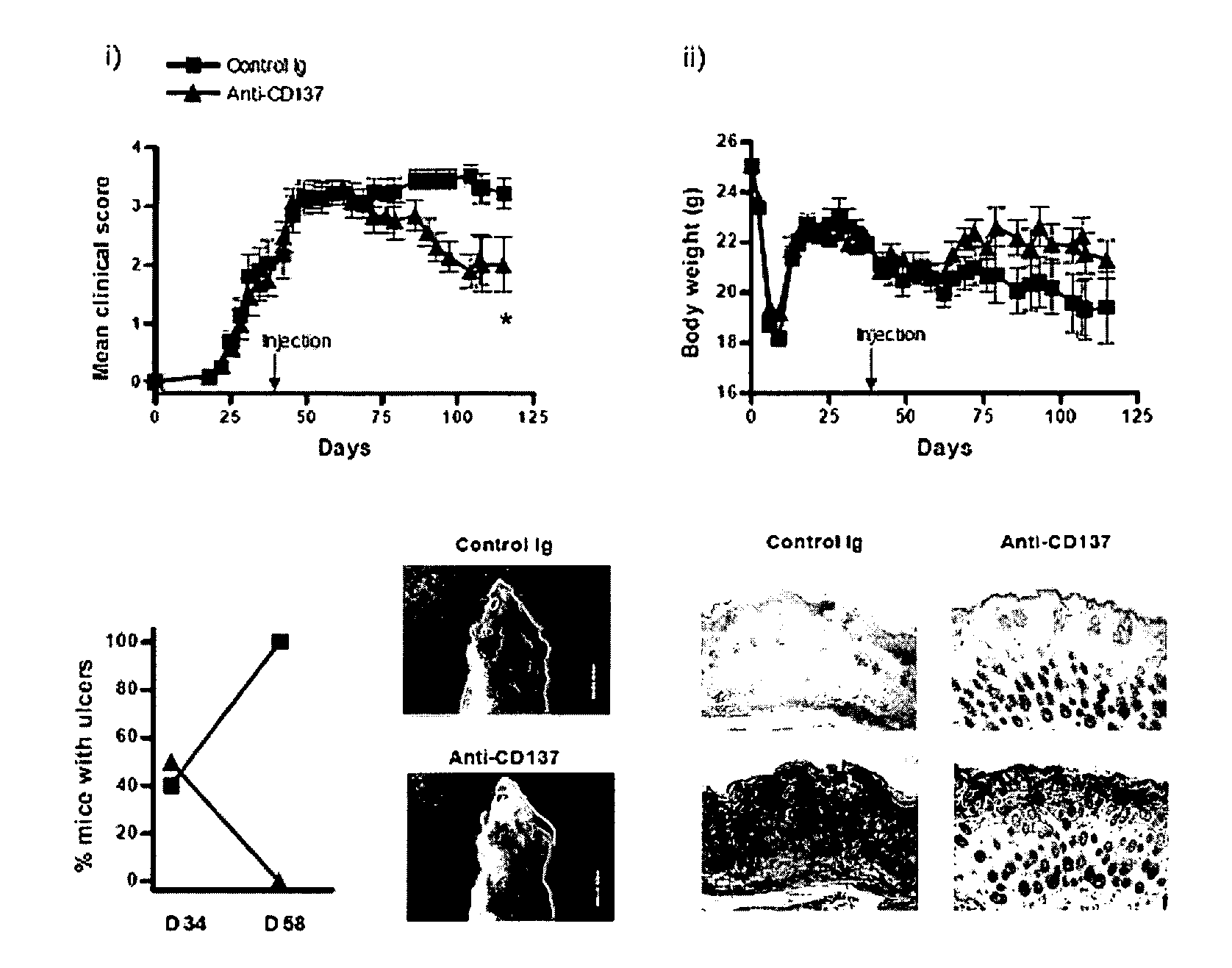
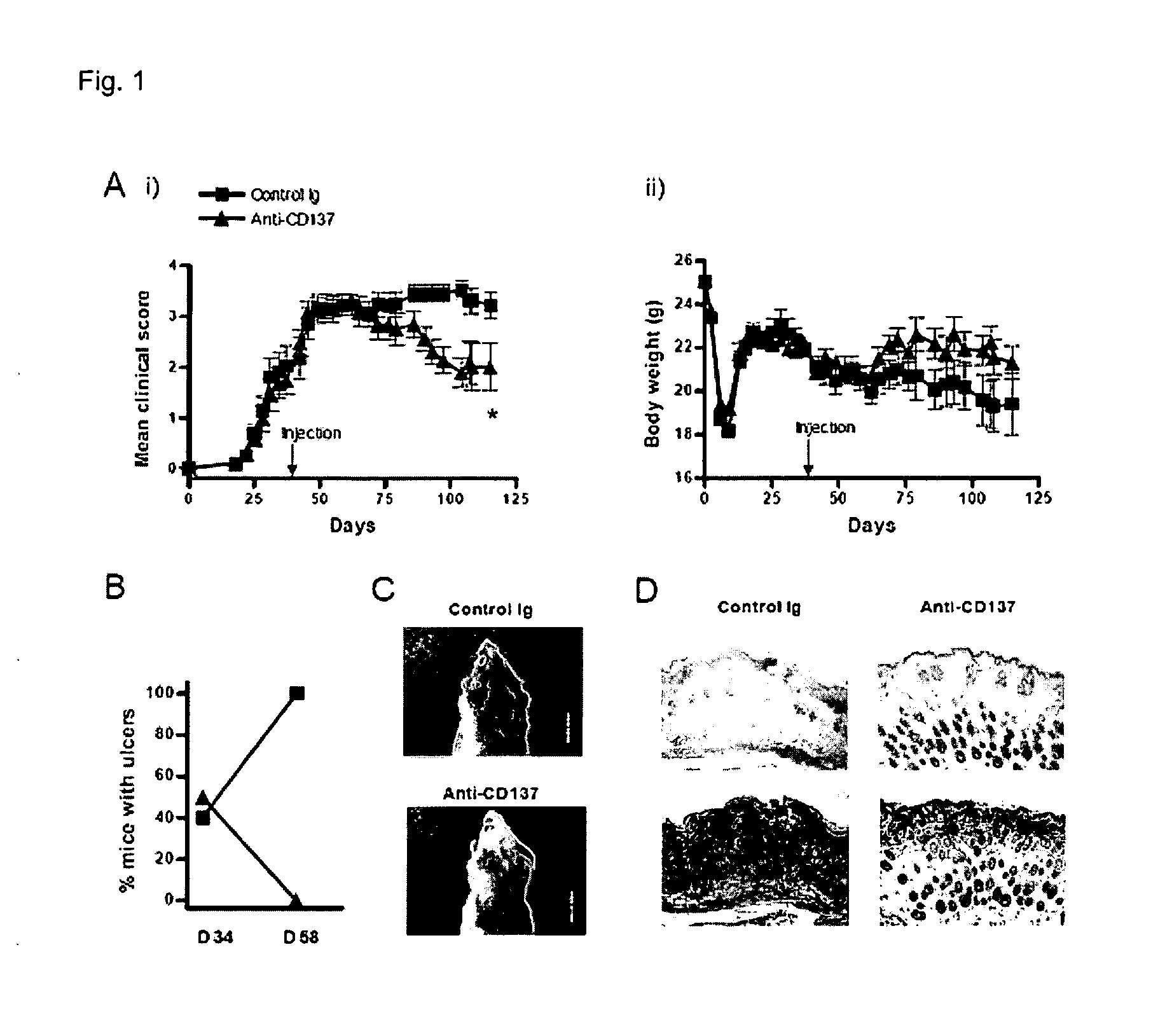
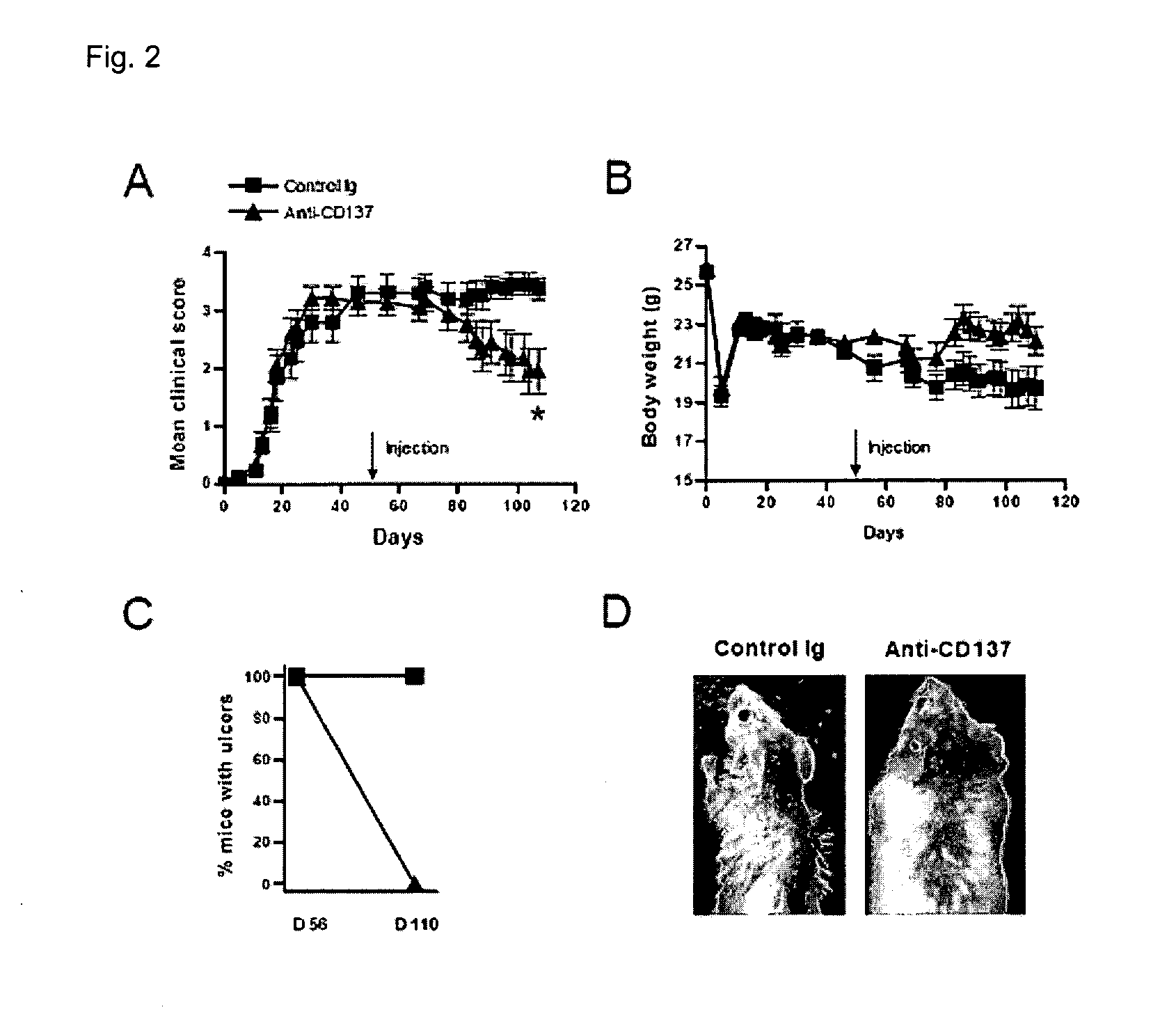
Pharmaceutical Composition for Preventing or Treating Chronic Graft-Versus-Disease Comprising Anti-CD137 Monoclonal Antibody
Provided is a pharmaceutical composition for preventing and treating chronic graft-versus-host disease (cGVHD) containing an anti-CD137 monoclonal antibody.The pharmaceutical composition containing the anti-CD137 monoclonal antibody as an active component reduces a cytokine produced from a CD4+ T cell, and increases a death of a donor CD4+ T cell, thereby effectively preventing and treating cGVHD, and thus can be useful for allogeneic stem cell transplantation.
Owner:UNIV OF ULSAN FOUND FOR IND COOPERATION
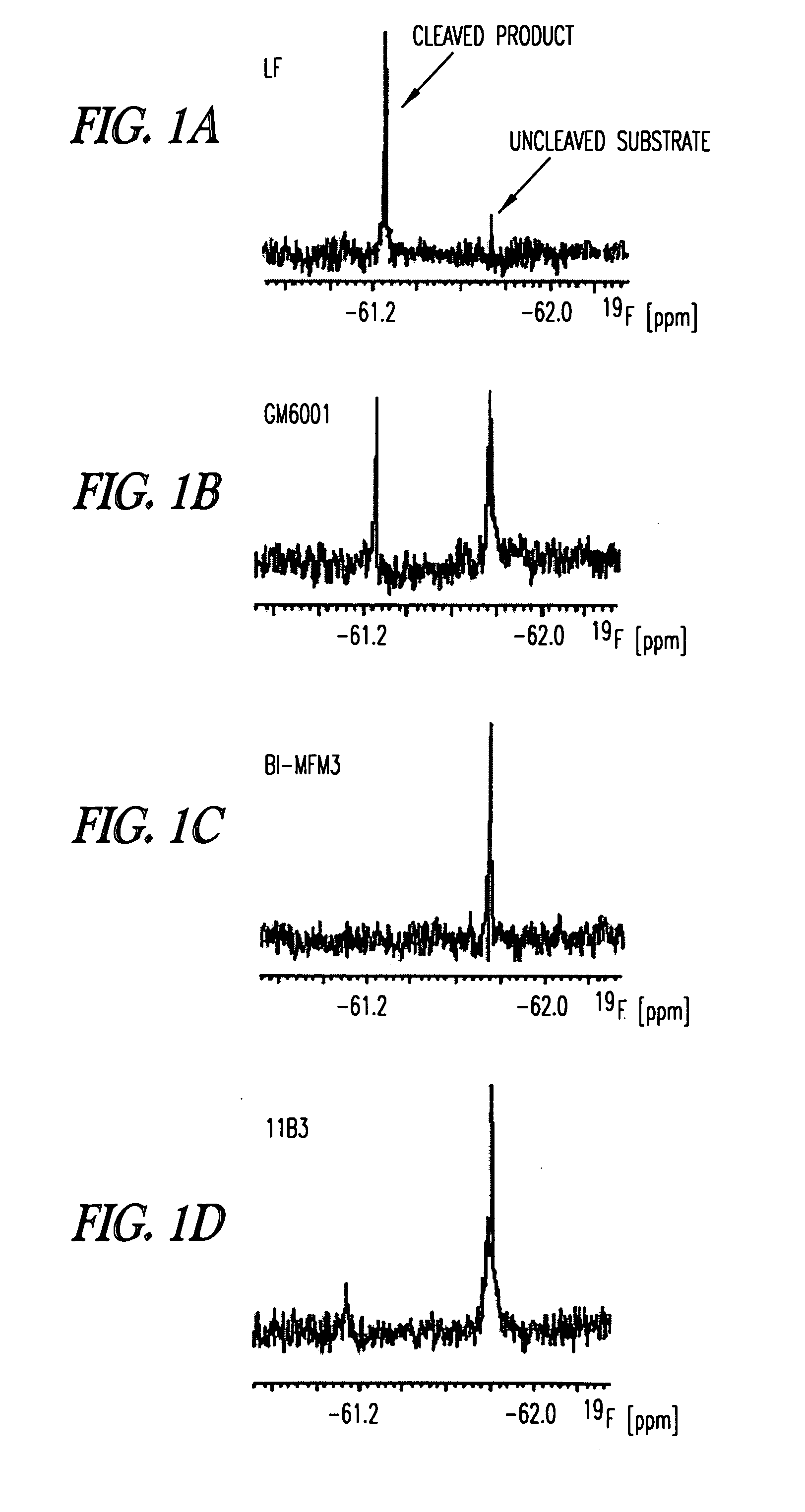
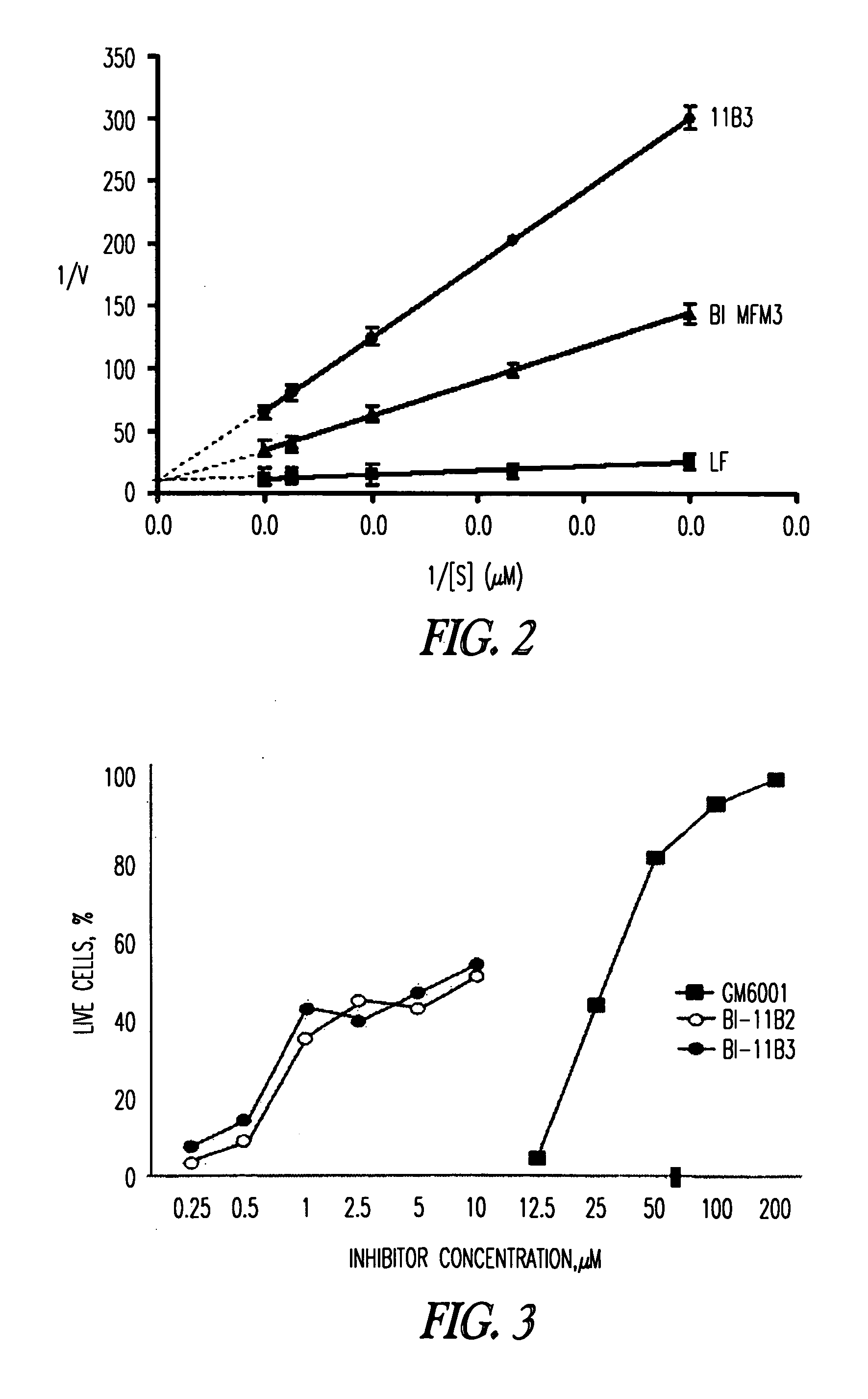
Inhibition of lethal factor protease activity from anthrax toxin
The present invention provides compounds that efficiently and specifically inhibit lethal factor (LF) protease activity of anthrax toxin.
Owner:SANFORD BURNHAM PREBYS MEDICAL DISCOVERY INST
Technique for preparing amalgamation protein skin-protection product containing albuminar and skin cell growth factor, and uses of the same
ActiveCN101172091AIncrease productionEasy to grow on a large scaleCosmetic preparationsPeptide/protein ingredientsWrinkle skinFactor ii
The present invention provides a recombinant fusion protein which stimulates the rejuvenation and reactivation of skin and epidermal cells for improving skin appearance, smoothing wrinkles and freckles, and whitening skin. Particularly, the present invention provides various types of products for improving skin, which contain recombinant fusion protein of human serum albumin (HSA) with cytokine peptides (EGF, FGF, KGF, HGH, HGF, PDGF, GCSF, interferon, IL-11 or IGF) by genetic engineering technology. The fusion protein can be used independently or in a combination or combination with yeast fermentation products, or with varied emulsifiers, thickeners, moisturizer, preservatives, yeasts and ferments.
Owner:BEIJING MEIFUYUAN BIO PHARM TECH +2
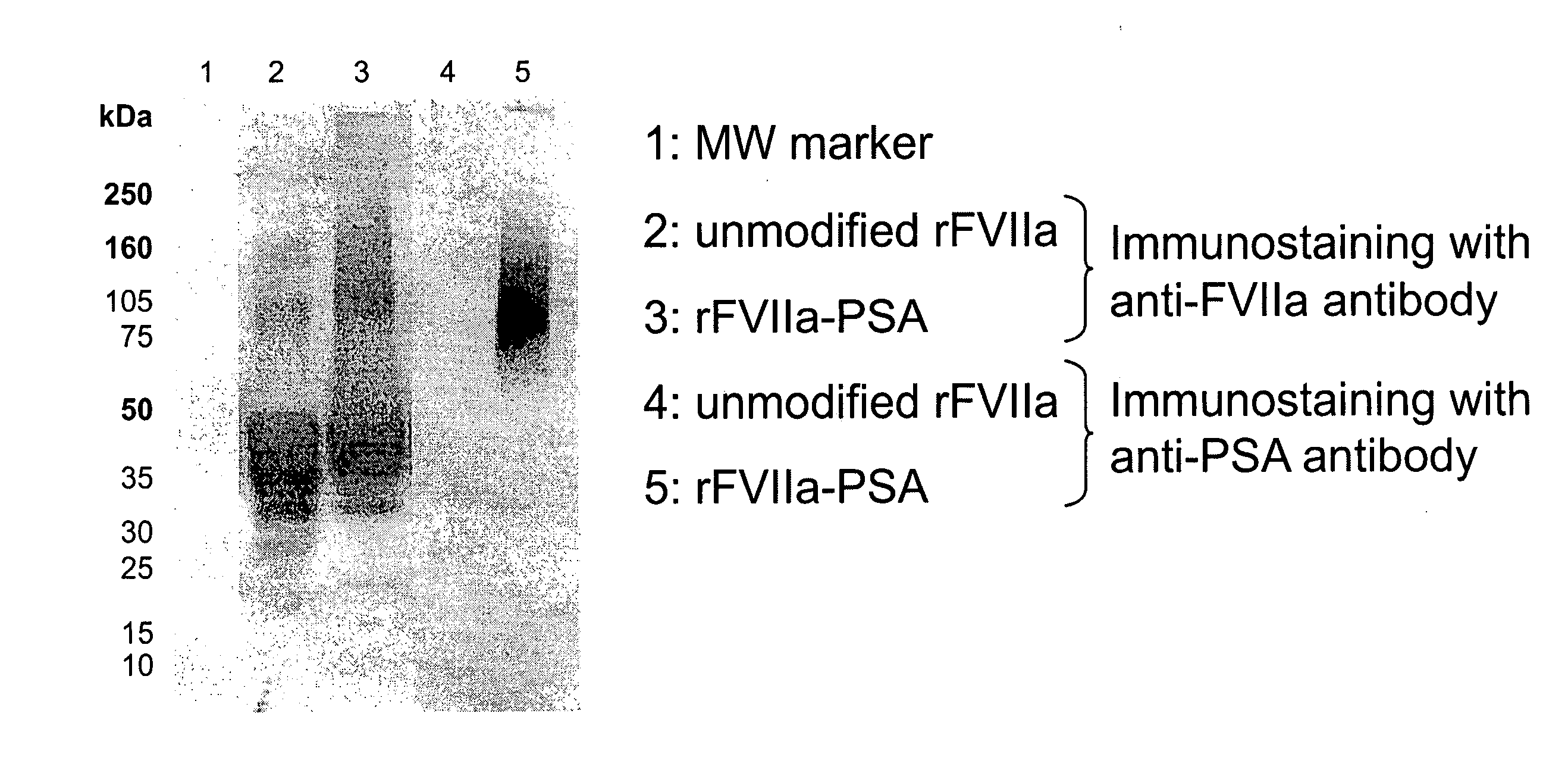
Factor VIIa-Polysialic Acid Conjugate Having Prolonged In Vivo Half-Life
ActiveUS20080221032A1Control bleedingProlong half-life in vivoPeptide/protein ingredientsPeptide preparation methodsFactor VIIaHalf-life
The present invention relates to a proteinaceous construct comprising plasmatic or recombinant factor VIIa (FVIIa) or biologically active derivatives thereof, which are bound to a carbohydrate moiety comprising 1-4 sialic acid units, wherein the in vivo half-life of the proteinaceous construct is substantially prolonged in the blood of a mammal, as compared to the in vivo half-life of a FVIIa molecule not bound to a carbohydrate moiety. The invention also provides a method for controlling bleeding in a mammal having a bleeding disorder due to functional defects or deficiencies of FVIIa, FVIII, or FIX. The invention also provides a method for controlling bleeding in a mammal during surgery or trauma.
Owner:TAKEDA PHARMA CO LTD
Nanoparticles for providing immune responses against infectious agents
ActiveUS20090297614A1Strong immune responseConvenience needsPowder deliveryHeavy metal active ingredientsNanoparticleT cell
Nanoparticles for providing immune responses for the treatment or prophylaxis of infection by infectious agents such as viruses, parasites, bacteria, prions and fungi are described which comprises a core including metal and / or semiconductor atoms, wherein the core is covalently linked to a plurality of ligands, the ligands including a carbohydrate residue capable of stimulating an innate immune response, a T cell helper peptide and a danger signal. This platform may then be adapted by including one or more further ligands capable of producing a specific response to a target infectious agent.
Owner:MIDATECH LTD
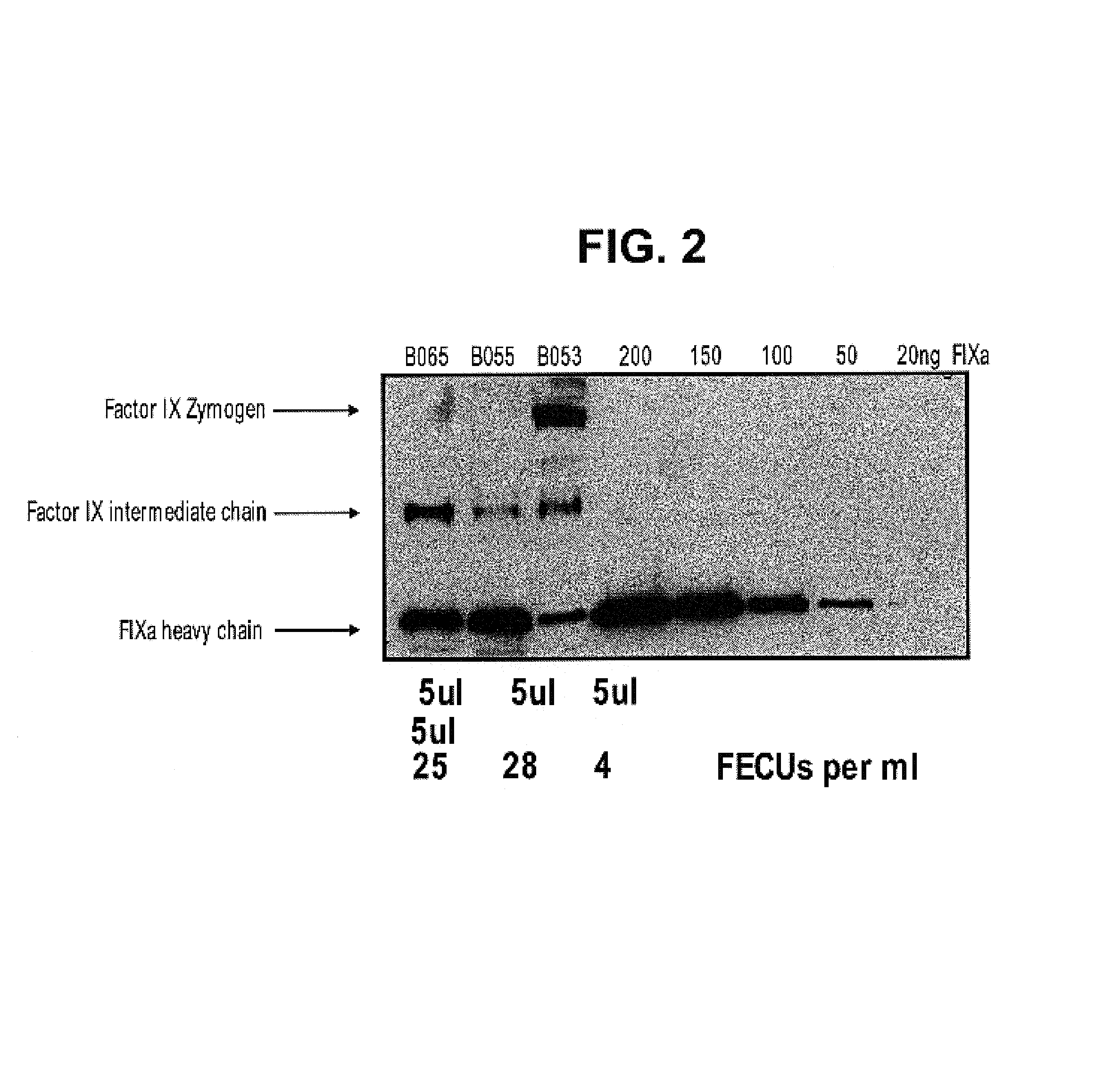
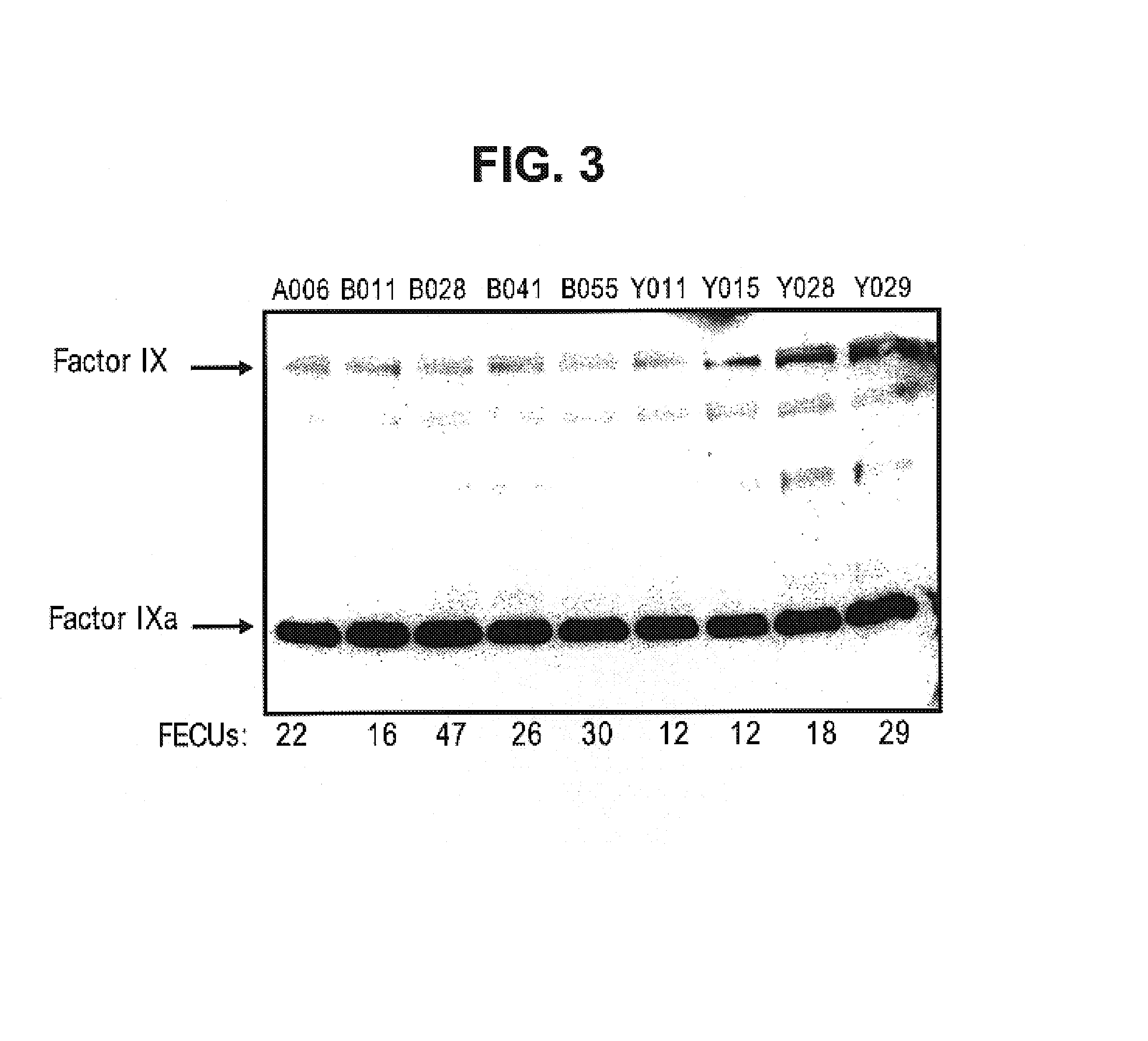
Factor IXa for the treatment of bleeding disorders
Owner:BAXALTA GMBH
Humanized heterogenous cell epimatrix material and preparation method thereof
The invention relates to a humanized heterogenous extracellular matrix material, which is a dried cellular foam material made by compounding an extracellular matrix and a cell growth factor which are synthesized and excreted by human body cells on a heterogenous extracellular matrix. Cells and natural antigen components are removed from the prepared humanized heterogenous extracellular matrix material, and when the humanized heterogenous extracellular matrix material is applied to the surface of wound, the humanized heterogenous extracellular matrix material can directly take part in the repair of the surface of wound, and the cell growth factor contained in the humanized heterogenous extracellular matrix material can guide the ingrowth of the cells around the wound and creation of blood vessel, and induce the differentiation of stem cells to skin cells, thereby obviously promoting the healing of the surface of wound; besides, the prepared product is a dried product, which greatly prolongs storage life, and not only has part of the characteristics of human body tissues and good mechanical property of the heterogenous extracellular matrix, but also has higher biocompatibility to human body and obviously reduces the immune exclusive reaction; at the same time, the prepared product can cover the surface of wound, fill the defection of soft tissues, promote the growth and proliferation of the cells around the wound, repair the defection of the soft tissue organs, and promote the wound healing.
Owner:SHAANXI RUISHENG BIOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com