Compositions, devices and methods for factor VII therapy
A technology of composition and polymer, applied in the field of composition, device and method for factor VII therapy, capable of solving problems such as short half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0427] Example 1: Generation and Culture of Exemplary Engineered ARPE-19 Cells
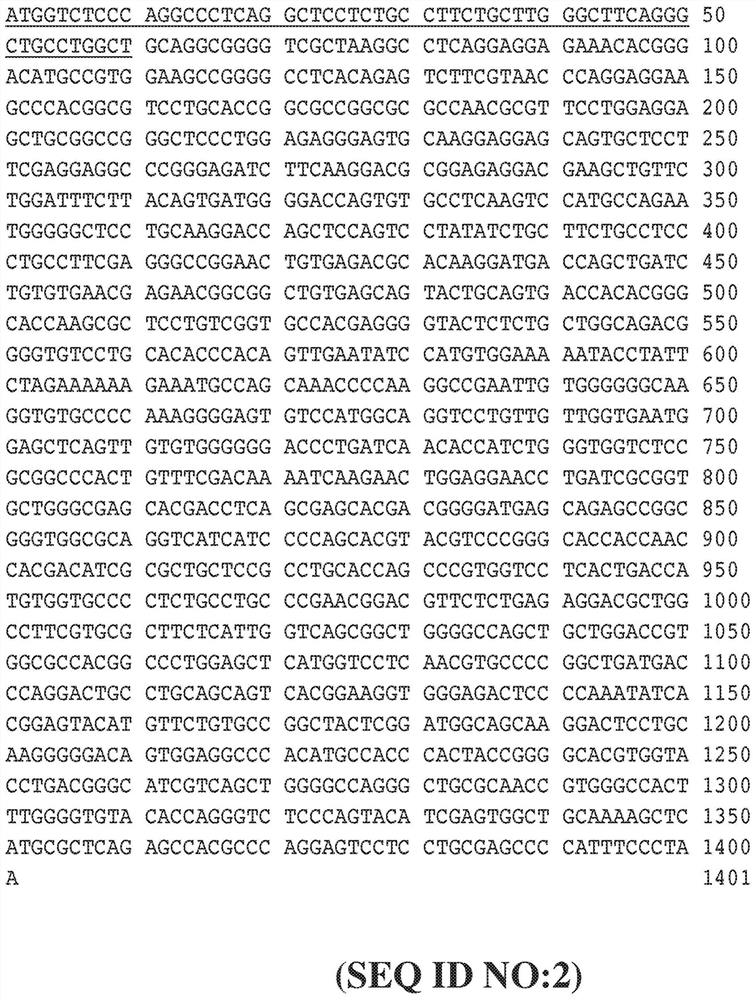
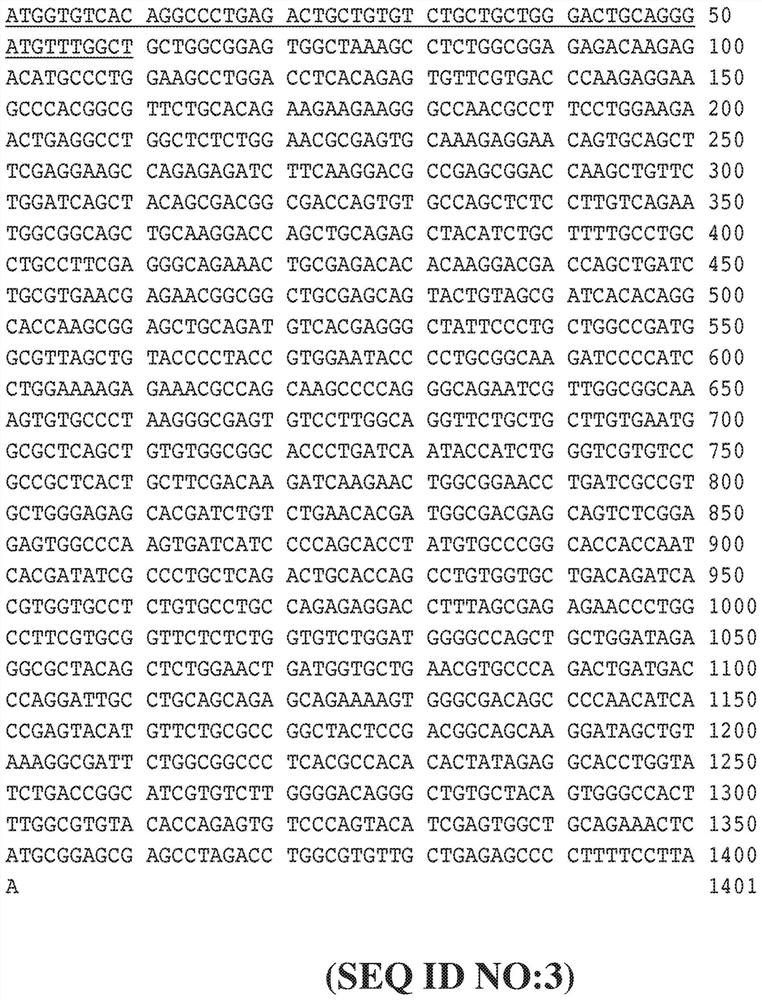
[0428] use Figure 8 The indicated expression vectors generate FVII secreting cells into which a foreign nucleotide sequence encoding a precursor FVII protein has been inserted using standard cloning methods. To achieve this, ARPE-19 cells were co-transfected with PiggyBac containing the transposase plasmid together with the FVII expression vector, and stably transfected cells were cultured in complete growth medium containing puromycin. The FVII expression vector was identical to the vector in Figure 6, except that one or two transcription units were inserted between the 5'ITR and 3'ITR. In addition to the promoter sequence and the inserted FVII coding sequence or FVII albumin fusion sequence, the backbone sequence of each transcription unit is Figure 6A and Figure 6B The corresponding sequences shown in are identical, for example, each transcription unit has the same Kozak sequence and the s...
example 2
[0438] Example 2: FVII secretion from exemplary engineered ARPE-19 cells
[0439] To quantify FVII protein secretion by cells, polyclonal populations of ARPE-19 cells engineered with the different FVII expression constructs described in Example 1 were trypsinized as described above and 500,000 cells were plated in 6-well tissue Place 2 ml of growth medium in a single well of a Petri dish. The engineered cells were allowed to settle and attach to the dish for 3-4 hours before the growth medium was replaced with fresh medium. One day (24 hours) after the medium change, the growth medium containing secreted FVII was collected and placed on ice. Cells were washed with phosphate-buffered saline and trypsinized with 0.05% trypsin EDTA as previously described. Cells were harvested and counted using trypan blue on a Countess II cell analyzer. FVII protein levels of the engineered cells described above were determined using conditioned growth medium and run on a commercially availab...
example 3
[0440] Example 3: Synthesis of Exemplary Compounds of Formula (I)
[0441] General plan
[0442] The following procedures describe methods for preparing exemplary compounds for use in the implantable device preparations described herein. The compounds provided herein can be prepared from readily available starting materials using modifications of the specific synthetic schemes described below that are well known to those skilled in the art. It will be appreciated that where typical or preferred process conditions (ie, reaction temperatures, times, mole ratios of reactants, solvents, pressures, etc.) are given, other process conditions can also be used unless otherwise stated. Optimum reaction conditions may vary with the particular reactants or solvent used, but such conditions can be determined by one skilled in the art by routine optimization procedures.
[0443] Furthermore, as will be apparent to those skilled in the art, conventional protecting groups may be necessary t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com