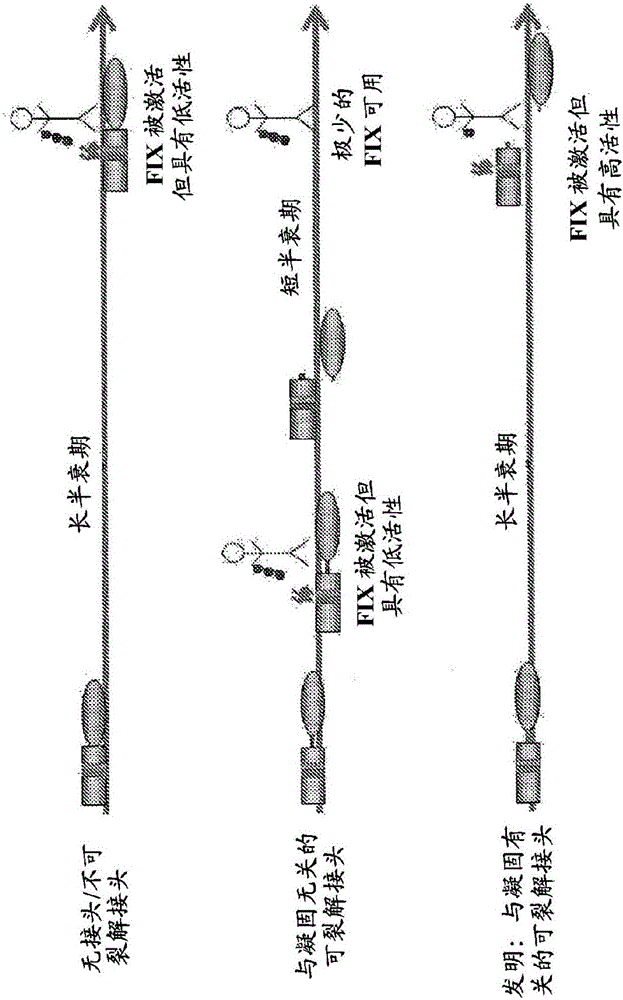
Fusion proteins comprising factor ix for prophylactic treatment of hemophilia and methods thereof
A fusion protein, hemophilia technology, applied in the direction of serum albumin, coagulation/fibrinolytic factor, plasma life extension fusion, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
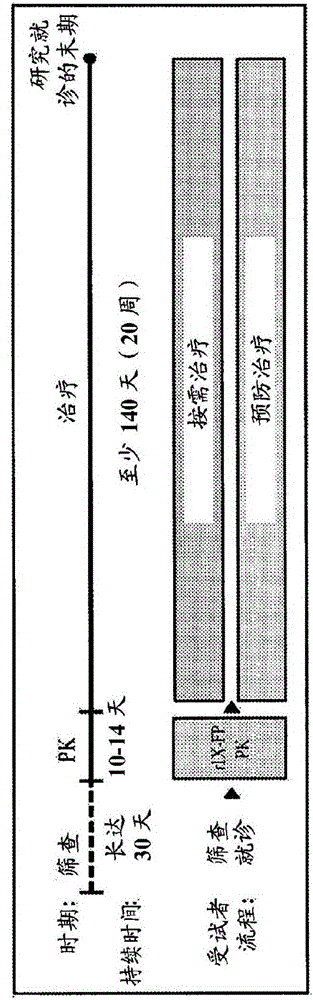
[0182] Example 1: Phase I / II open-label trial of the safety and efficacy of a novel recombinant fusion protein linking coagulation factor IX to albumin (rIX-FP) in patients with hemophilia B
[0183] This trial was designed to evaluate the efficacy of rIX-FP for the prevention of bleeding episodes during weekly prophylaxis and to evaluate the hemostatic efficacy for the treatment of bleeding, as well as to evaluate the safety and pharmacokinetics of rIX-FP ( PK).
[0184] patients and methods
[0185] patient
[0186] The criteria for selecting subjects were based on the draft Guidelines on the clinical investigation of recombinant and human plasma-derived factor IX products of the Committee on Human Medicine (European Medicines Agency, Committee on Human Medicine (CHMP), Guideline in the Clinical Investigation of Recombinant and Human Plasma-Derived Factor IX Products.2009.CHMP / BPWP / 144552 / 2009. Available here: http: / / www.ema.europa.eu / docs / en_GB / document_library / Scientif...
Embodiment 2
[0244] Example 2: Phase 3b open-label, multicenter, safety and efficacy extension study of recombinant coagulation factor IX albumin fusion protein (rIX-FP) in hemophilia B subjects
[0245] 1. Research overview
[0246]A prospective, open-label study evaluating the long-term safety and efficacy of rIX-FP was developed for bleeding event prophylaxis in hemophilia B subjects. The study will include, but is not limited to, study subjects enrolled in our previous Phase II / III and Phase III clinical studies. In addition, subjects requiring major non-emergency surgery who have not previously completed a CSL-funded rIX-FP lead-in study may be enrolled. At the end of the study, subjects from the lead-in study are expected to have accumulated at least 100 rIX-FP exposure days during enrollment in all CSL-funded rIX-FP studies.
[0247] The extension study consists of a prophylaxis treatment period of approximately 3 years, during which subjects will be administered rIX-FP as routine...
Embodiment 3
[0339] Example 3: Phase 3b Study of Recombinant Coagulation Factor IX Albumin Fusion Protein (rIX-FP) at 21 Day Dosing Intervals in Hemophilia B Subjects
[0340] Subjects ≥18 years of age may also receive rIX-FP as routine prophylaxis at doses up to 100 IU / kg with a 21-day treatment interval. Initially, they had to undergo a PK assessment period with a single injection of rIX-FP (100 IU / kg) after a rIX-FP washout period of at least 14 days.
[0341] In order to participate in the 21-day treatment program, the following criteria must be met:
[0342] 1. Subjects must be at least 18 years old.
[0343] 2. Completed at least 6 months of rIX-FP routine prophylaxis with a 14-day treatment interval regimen in Phase II / III or Phase III studies.
[0344] 3. Completed the 21-day pharmacokinetic evaluation of 100IU / kg rIX-FP.
[0345] In addition, investigators should evaluate the following before changing treatment intervals:
[0346] 1. Efficacy and safety
[0347] 2. Subject's ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com