A kind of carrimycin freeze-dried powder preparation and preparation method thereof
A technology of freeze-dried powder injection and carrimycin, which is applied in the field of medicine, can solve the problems of low bioavailability, and achieve the effects of short reconstitution time, outstanding curative effect, and less insoluble particles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1: Screening and optimization of freeze-dried powder for injection
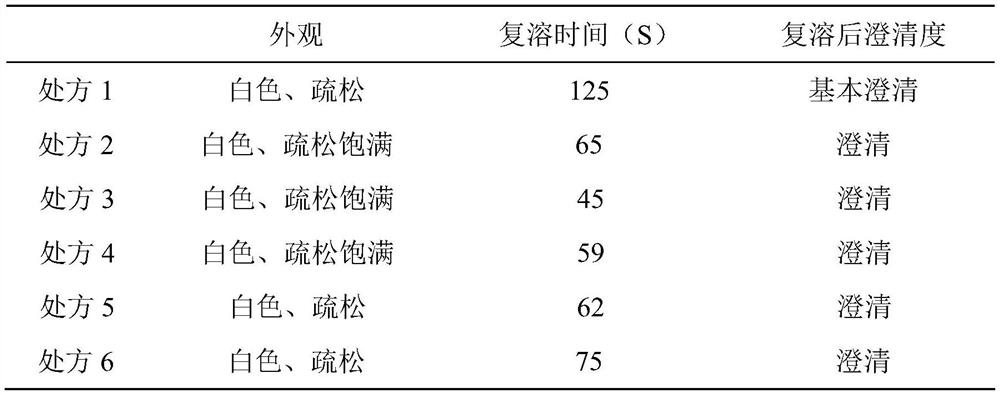
[0039] Appearance and Clarity: Visually inspect the appearance; dissolve the freeze-dried powder injection with sodium chloride injection before checking, and visually inspect its clarity.
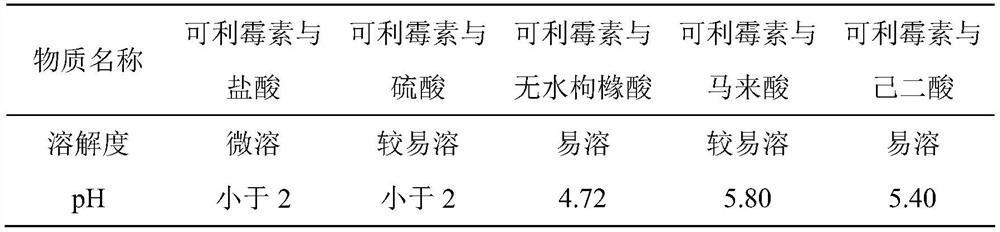
[0040] (1) Comparison of the solubility of carrimycin and organic or inorganic acids in water
[0041]
[0042] The above solubility test is based on the solubility of 100 mg of carrimycin in water at 25 °C as an indicator;
[0043] Slightly soluble: 100mg of carrimycin is dissolved in 10-100ml of water;
[0044] More soluble: 100mg of carrimycin is dissolved in 2-3ml of water;
[0045] Soluble: 100mg of carrimycin is dissolved in 0.1-1ml of water;
[0046] The test results show that the solubility of canrimycin is improved to different degrees after mixing with different acids. The solubility of mycin component is better, but when canrimycin is combined with sulfuric acid, its pH is less than 2 and ...
Embodiment 2
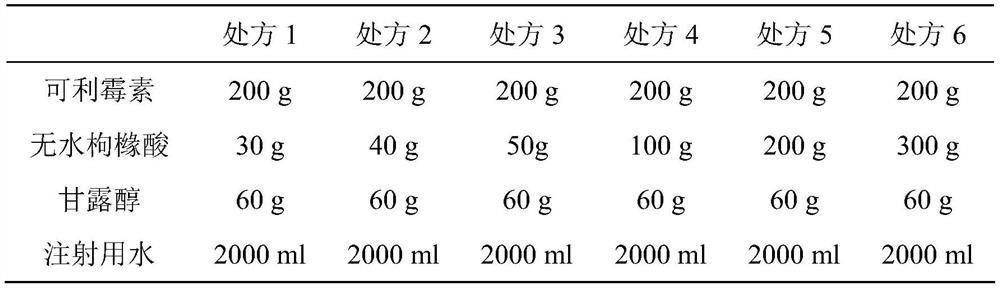
[0089] prescription:
[0090]
[0091] Preparation method: take a certain proportion of the prescribed amount of water for injection, add the prescribed amount of mannitol, after it is completely dissolved, add the prescribed amount of anhydrous citric acid and karimycin, and add the remaining amount of water for injection, wait until it is completely dissolved. After dissolving, the intermediate medicinal liquid is obtained, which is then filtered through a filter membrane. Put it in a freeze-drying box, the temperature is -40℃, pre-freeze for 5h, the sublimation temperature is -20℃, the vacuum is ≤20Pa, the sublimation time is about 10h, and then it is heated and dried, the drying temperature is 10℃, the time is 4h, and the temperature is 25°C for 4 hours.
Embodiment 3
[0093] prescription:
[0094]
[0095] Preparation method: take a certain proportion of the prescribed amount of water for injection, add the prescribed amount of mannitol, after it is completely dissolved, add the prescribed amount of anhydrous citric acid and karimycin, and add the remaining amount of water for injection, wait until it is completely dissolved. After dissolving, the intermediate medicinal liquid is obtained, which is then filtered through a filter membrane. Put it in a freeze-drying box, the temperature is -40℃, pre-freeze for 5h, the sublimation temperature is -20℃, the vacuum is ≤20Pa, the sublimation time is about 10h, and then it is heated and dried, the drying temperature is 10℃, the time is 4h, and the temperature is 25°C for 4 hours.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com