A kind of stephanine hydrochloride freeze-dried powder, preparation method and application thereof
A technology of salicylic acid hydrochloride and freeze-dried powder, which is applied in freeze-dried delivery, powder delivery, pharmaceutical formulation and other directions, can solve the problems of large dosage, decreased storage content, low active pharmaceutical ingredients, etc., and achieves high bioavailability, high The effect of reducing the frequency of medication and reducing the amount of insoluble particles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
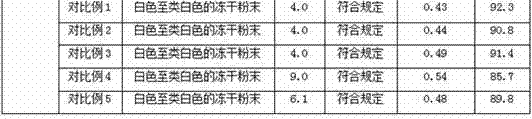
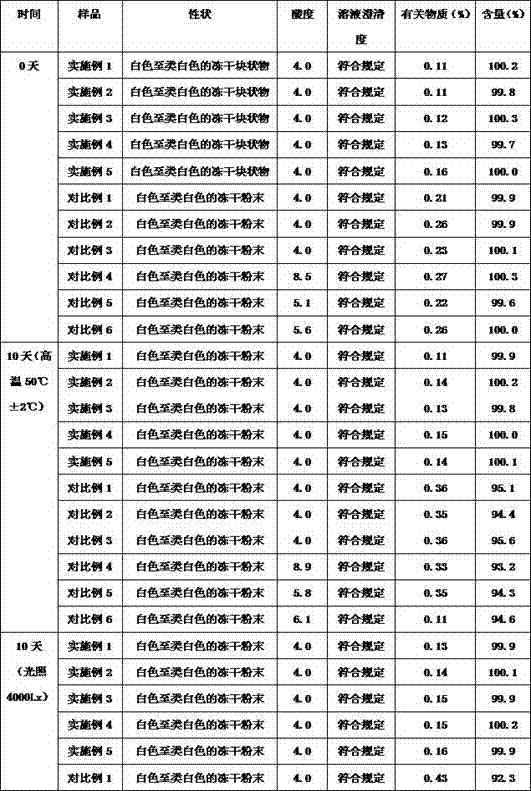
Image
Examples
Embodiment 1
[0022] Example 1: Lyophilized powder of stephenine hydrochloride
[0023] 1: In this example, the lyophilized powder of cephalanthine hydrochloride includes the following raw materials in weight: 13 g of cephalanthine hydrochloride, 20 g of trehalose, 26 g of lactose, and 100 g of sorbitol.
[0024] 2: The preparation method of the lyophilized powder of stephenine hydrochloride in this embodiment includes the following steps:
[0025] (1) Dissolve stephenine hydrochloride in water for injection at 70°C with 65% of the total solution and stir to dissolve;
[0026] (2) Add sorbitol, add trehalose and lactose after dissolution, and stir until completely dissolved;
[0027] (3) Add medicinal activated carbon, heat to 100°C for 20 minutes, filter, and add water for injection to the filtrate to the total amount of liquid preparation, the total amount of which is to add 6ml of water for injection per 1000 units of stephenine hydrochloride;
[0028] (4) Adjust the pH to 4;
[0029] (5) Filter wit...
Embodiment 2
[0031] Example 2: Lyophilized powder of stephenine hydrochloride
[0032] 1: In this example, the lyophilized powder of cephalothin hydrochloride includes the following materials: 10 g of cephalotine hydrochloride, 22 g of trehalose, 24 g of lactose, and 100 g of sorbitol.
[0033] 2: In this example, the preparation method of the lyophilized powder of stephenine hydrochloride is the same as that of example 1.
Embodiment 3
[0034] Example 3: Lyophilized powder of stephenine hydrochloride
[0035] 1: The lyophilized powder of cephaerine hydrochloride in this example includes the following raw materials in weight: 15 g of cephaerine hydrochloride, 18 g of trehalose, 28 lactose, and 100 g of sorbitol.
[0036] 2: In this example, the preparation method of the lyophilized powder of stephenine hydrochloride is the same as that of example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com