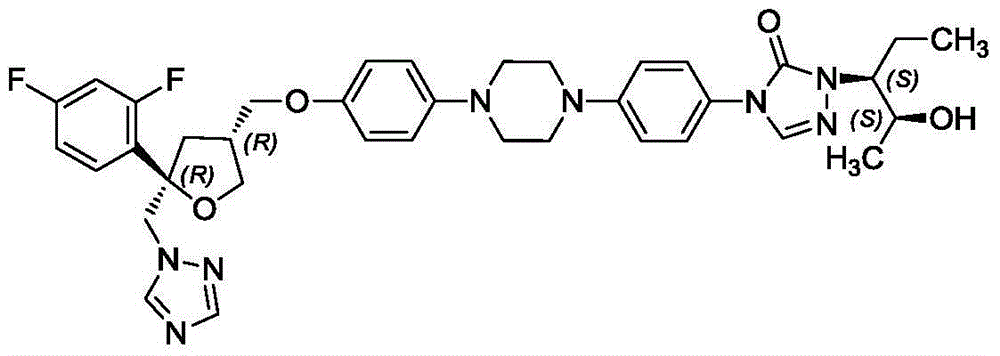
Preparation method for freeze-dried powder injection of posaconazole
A technology of posaconazole powder injection and freeze-dried powder injection, which is applied in the field of pharmaceutical preparations, can solve the problems of poor preparation stability, inconvenient transportation and storage, and environmental impact, so as to facilitate transportation and storage, satisfy storage and treatment, and avoid Effects of Environmental Pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
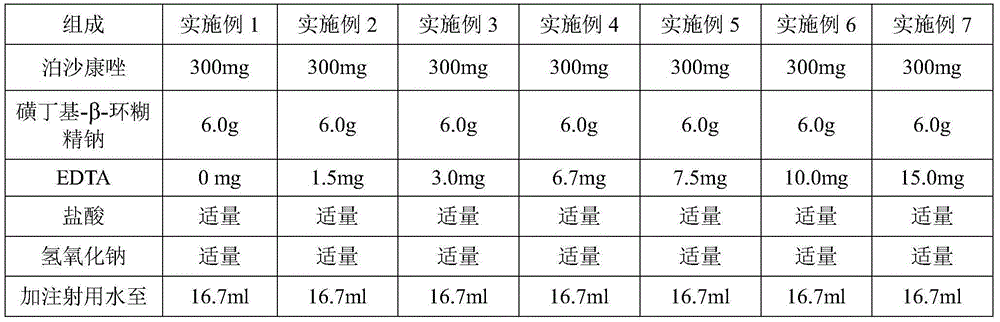
Embodiment 1-7
[0023]
[0024] Preparation Process:
[0025] (1) Take 1 / 3 to 2 / 3 of the prescription amount of water for injection, and dissolve edetate disodium and sulfobutyl-β-cyclodextrin in the batching tank.
[0026] (2) Adjust the pH of the solution to 1.4 with a pH regulator.
[0027] (3) Add posaconazole into the solution and stir to dissolve completely.
[0028] (4) Add a pH regulator to adjust the pH to 2.5, and add water for injection to make it volume.
[0029] (5) The solution is aseptically filtered, subpackaged and freeze-dried to obtain the final product.
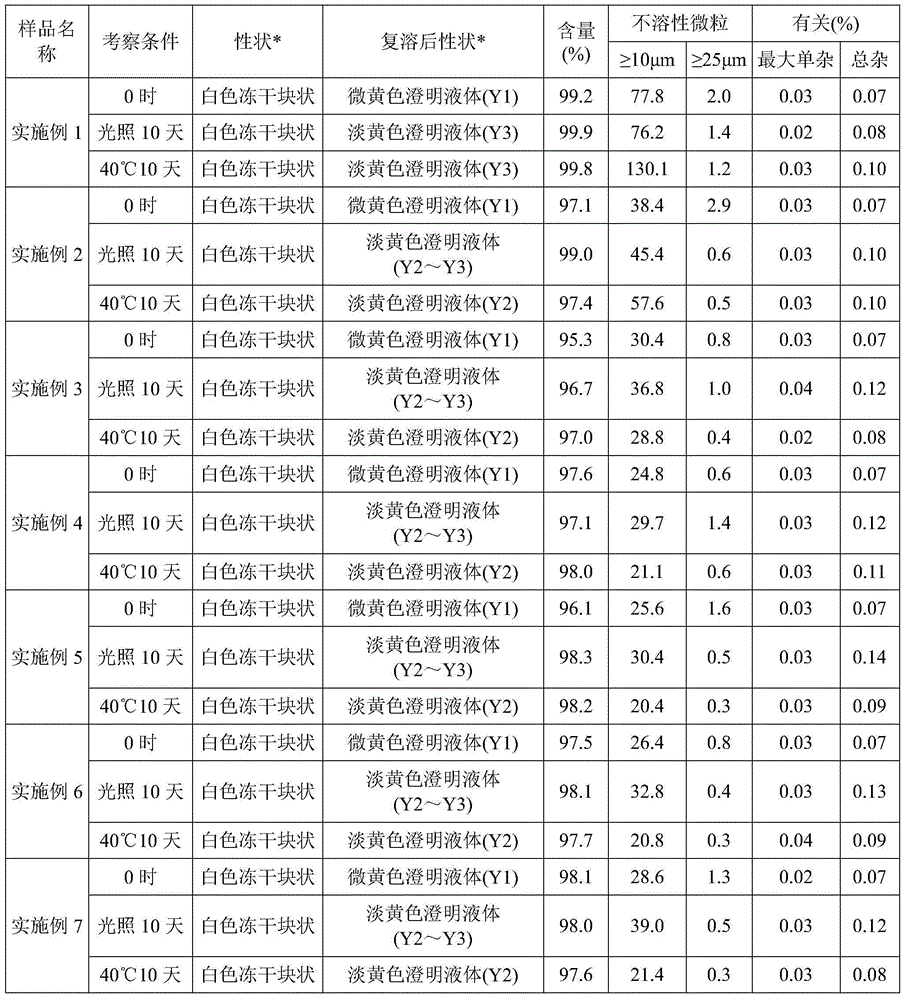
[0030] The posaconazole freeze-dried powder injection samples prepared in the above Examples 1 to 7 were subjected to a stability test, as shown in Table 1:
[0031] Table 1 posaconazole freeze-dried powder preparation stability test results
[0032]
[0033] Result: From the insoluble particle data before and after the compatibility of the sample at 0 o'clock, 10 days of light and 10 days at 40°C, it can be see...
Embodiment 8
[0035]
[0036] Preparation Process:
[0037] (1) Take 1 / 3 to 2 / 3 of the prescription amount of water for injection, dissolve edetate disodium and cyclodextrin in the batching tank
[0038] (2) Adjust the pH of the solution to 1.2 with a pH regulator.
[0039] (3) Add posaconazole to the solution and stir to dissolve completely
[0040] (4) Add pH regulator to adjust pH to 2.0, add water for injection to volume
[0041] (5) The solution is aseptically filtered, subpackaged, and freeze-dried to obtain.
Embodiment 9
[0043]
[0044] Preparation Process:
[0045] (1) Take 1 / 3 to 2 / 3 of the prescription amount of water for injection, dissolve edetate disodium and cyclodextrin in the batching tank
[0046] (2) Regulate the pH of the solution to 1.6 with a pH regulator
[0047] (3) Add posaconazole to the solution and stir to dissolve completely
[0048] (4) Add pH regulator to adjust pH to 2.2, add water for injection to volume
[0049] (5) The solution is aseptically filtered, subpackaged and freeze-dried to obtain the final product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com