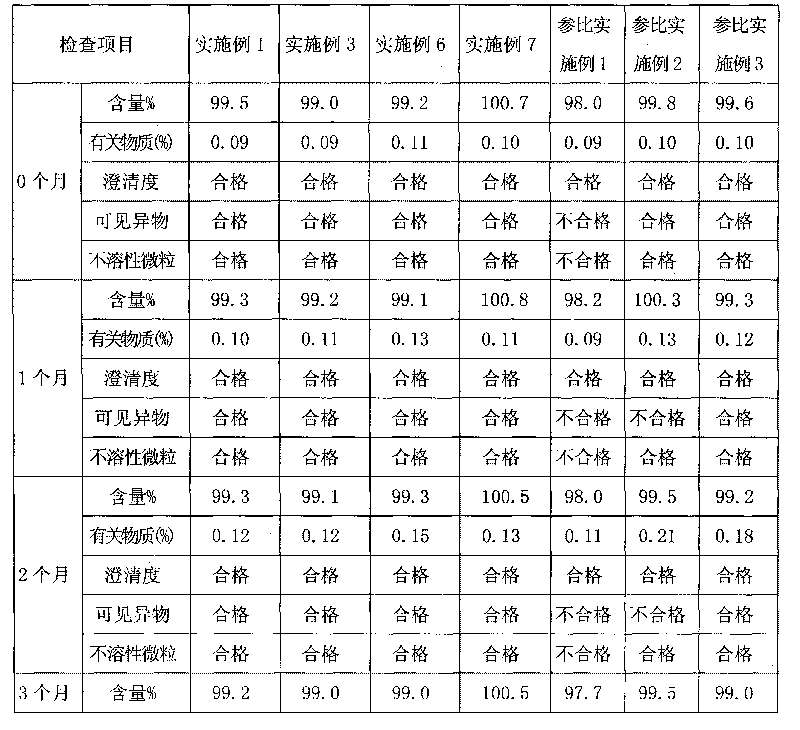
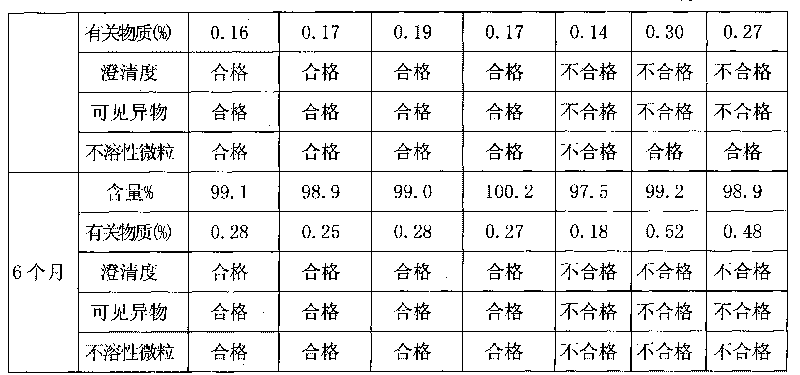
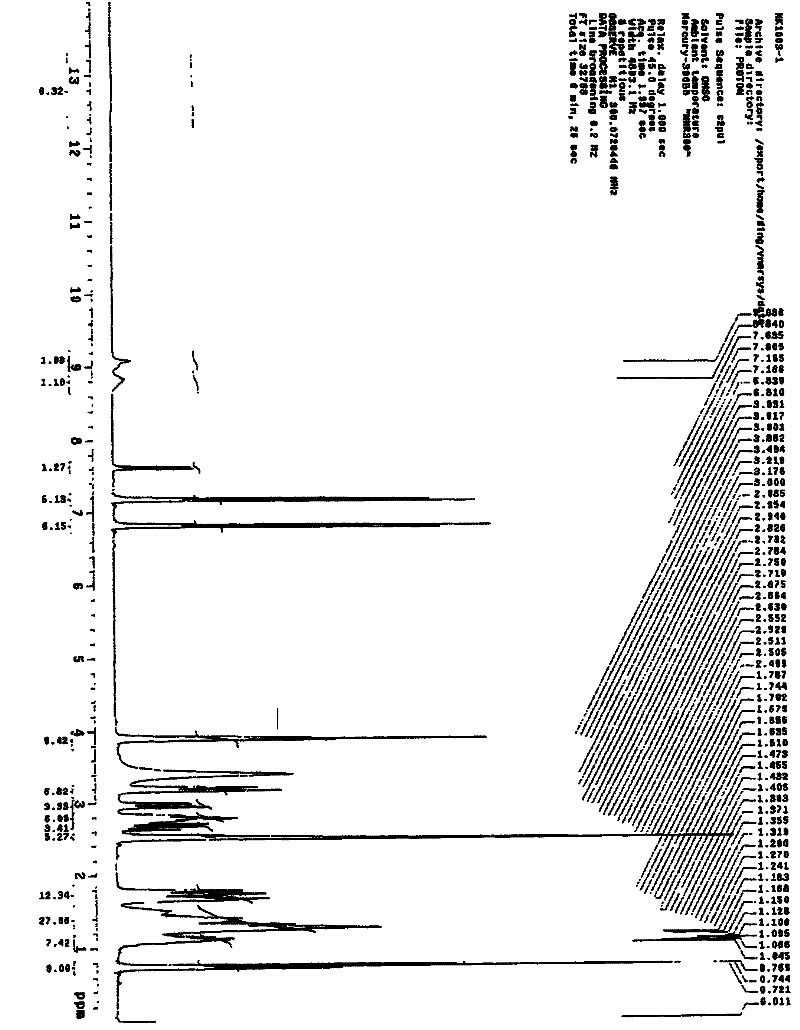
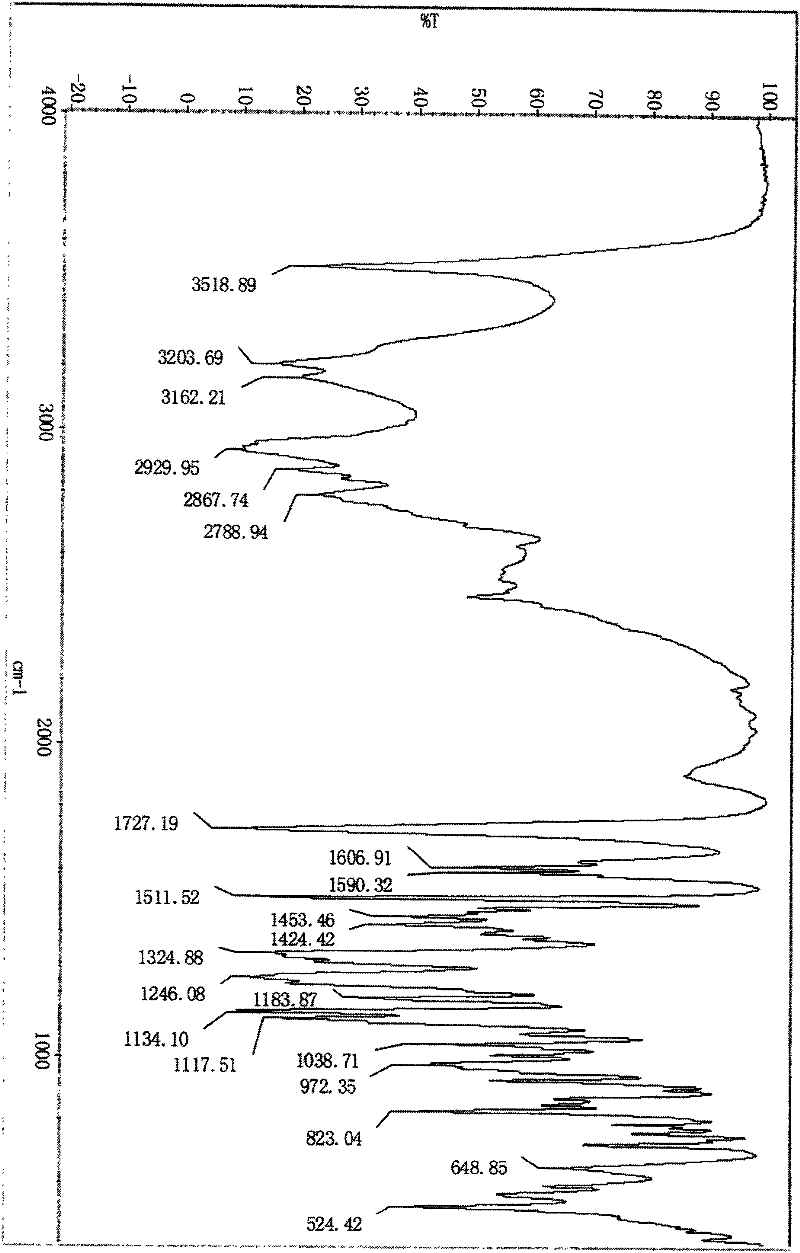
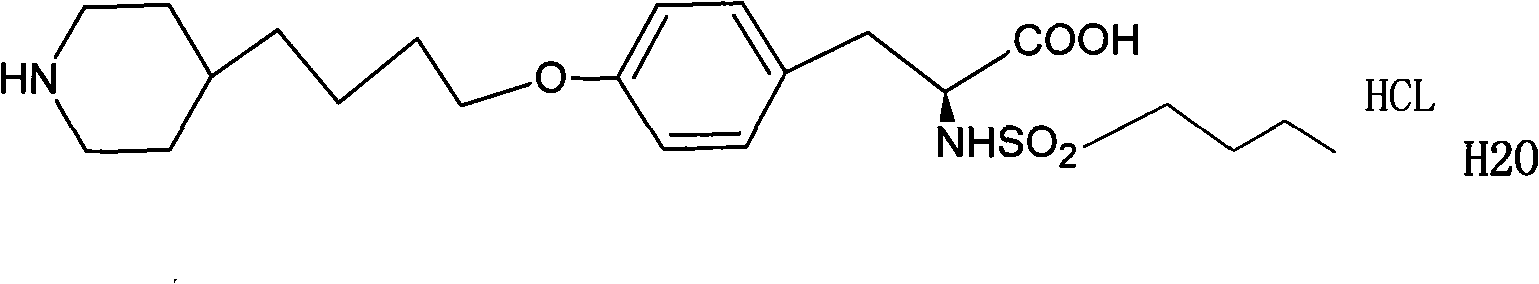
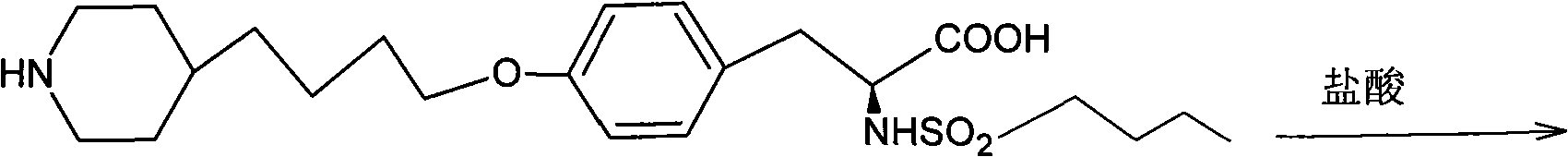
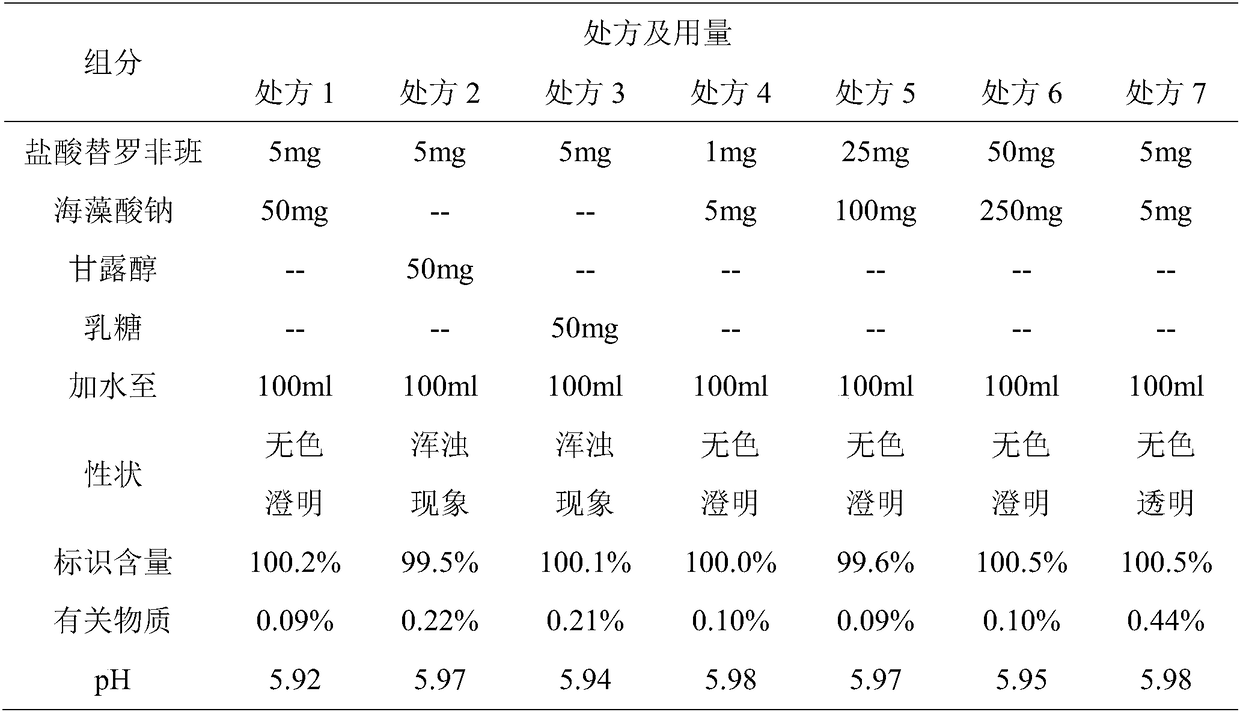
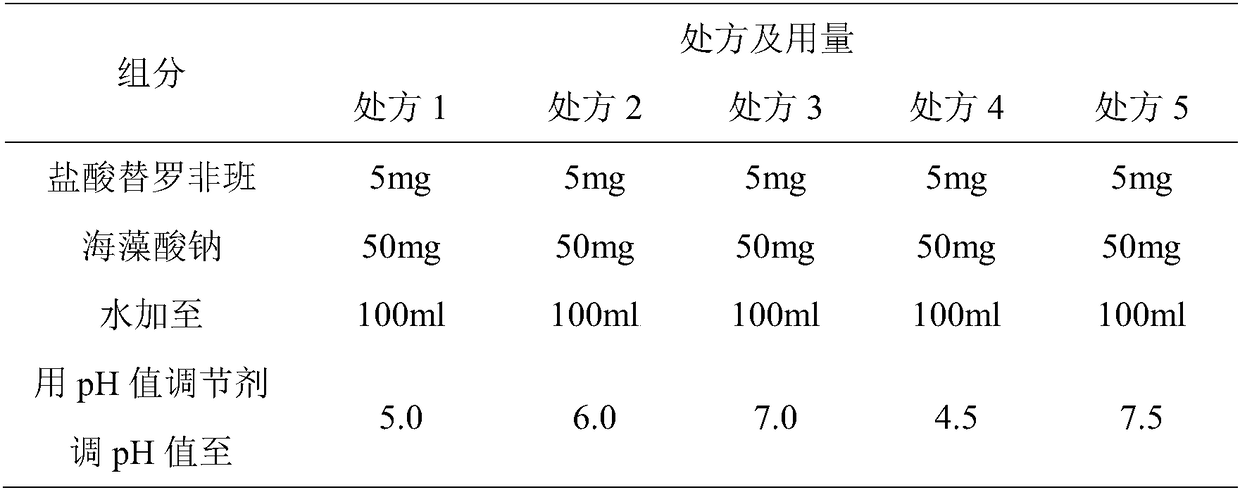
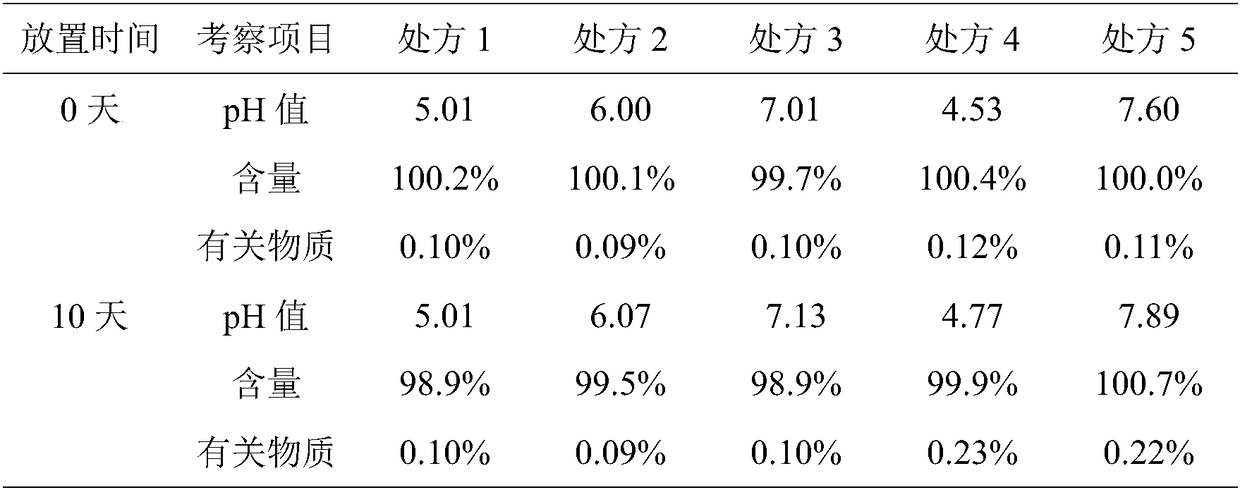
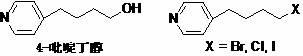Patents
Literature
58 results about "Tirofiban Hydrochloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
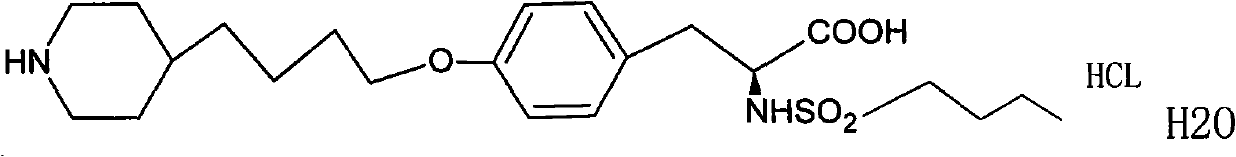
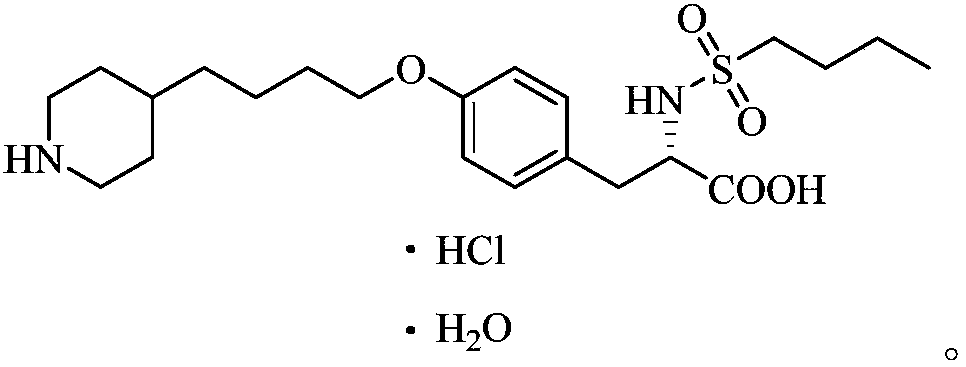
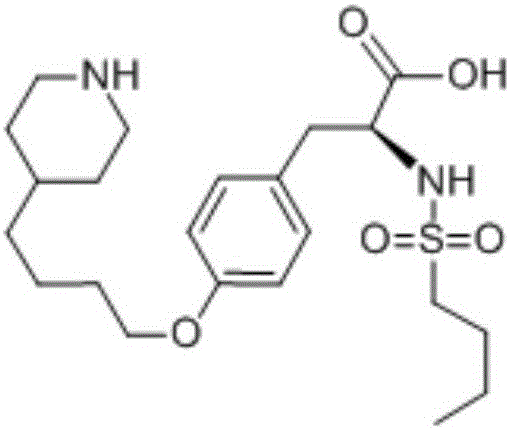
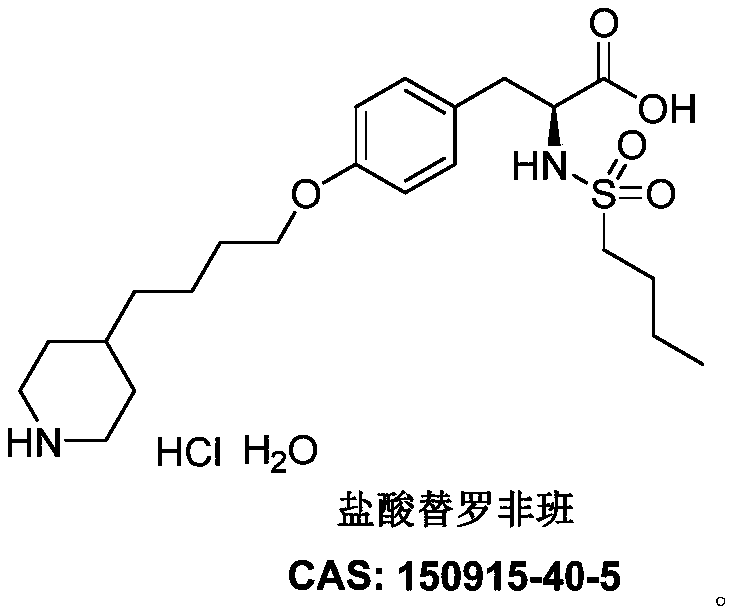
The hydrochloride salt form of tirofiban, a non-peptide tyrosine derivative with anticoagulant property. Tirofiban antagonizes fibrinogen binding to the platelet cell surface receptor, glycoprotein (GP) IIb/IIIA complex, one of the two purinergic receptors activated by ADP. The antagonism prevents adenylyl cyclase activation, which mediated via GP IIb/IIIa receptor complex, and results in decreased levels of cAMP and thereby interferes with platelet membrane function and subsequent platelet-platelet interaction, release of platelet granule constituents and prolongation of bleeding time.
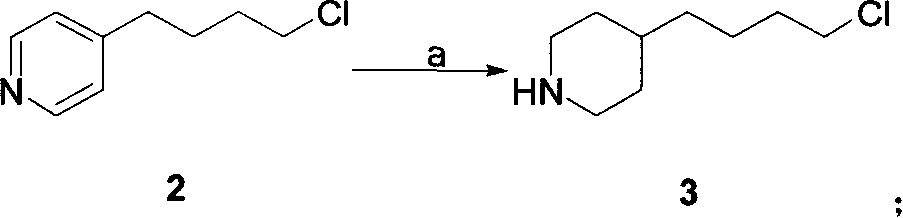
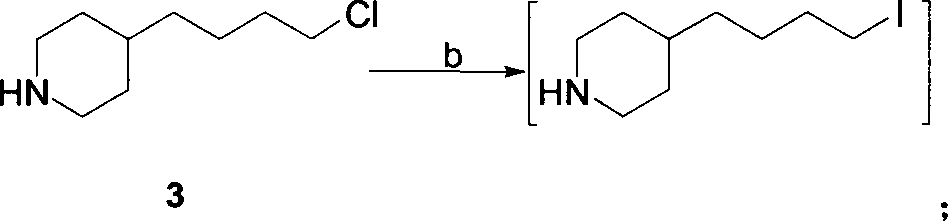
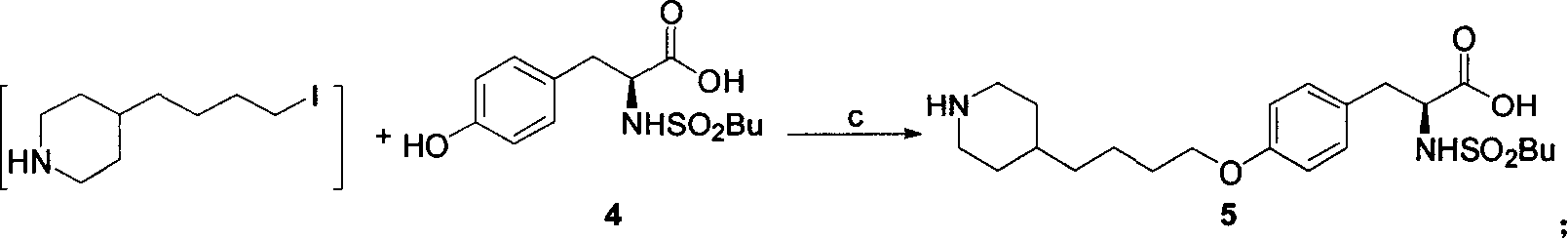
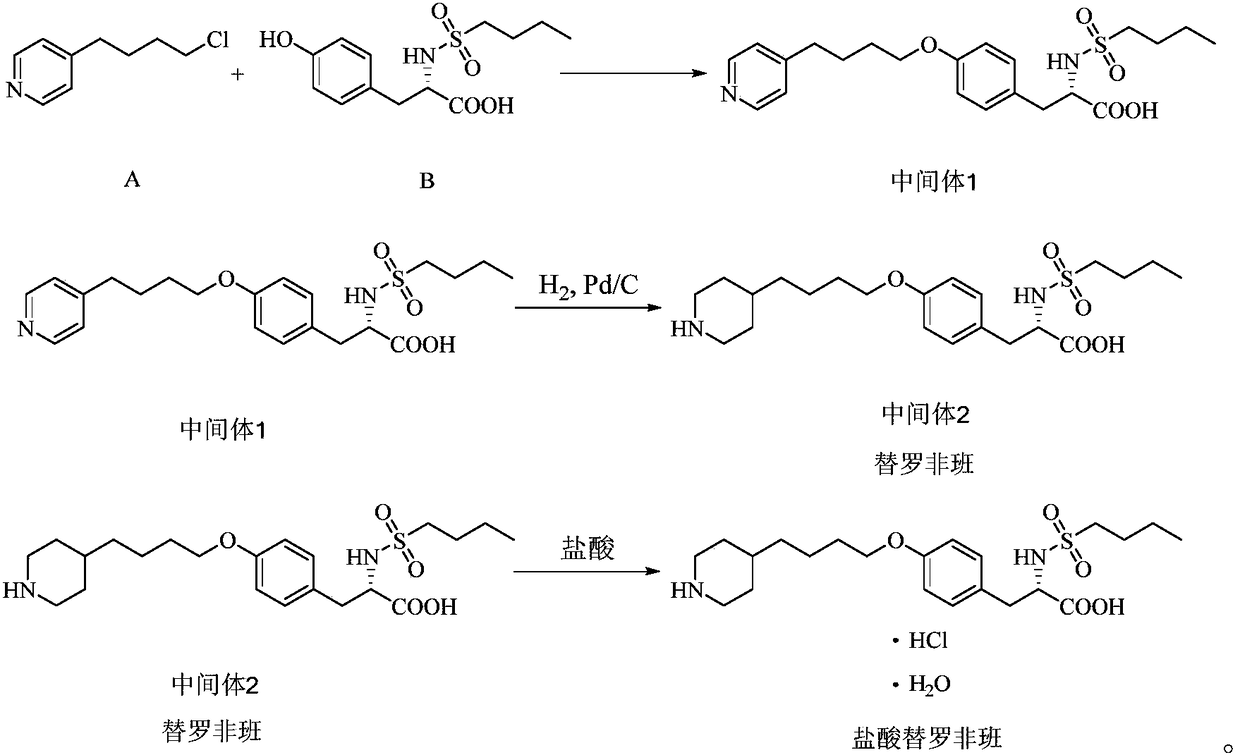
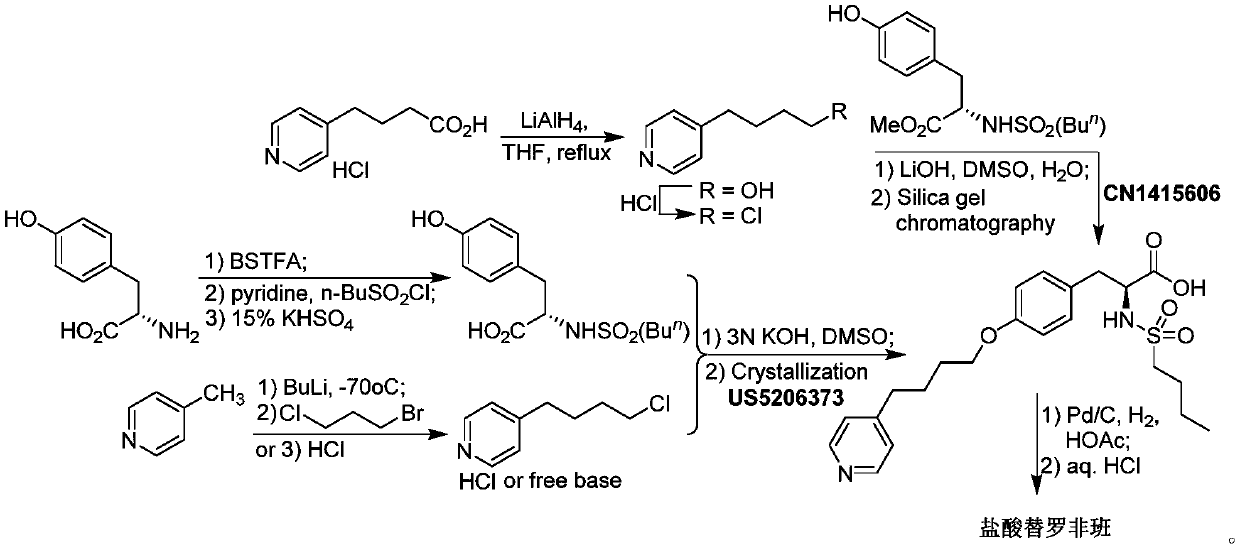
Process for preparation of tirofiban hydrochloride
ActiveCN1844099AHigh overall process yieldEasy to operate without dangerOrganic chemistryTirofiban HydrochlorideChemistry
The invention discloses a process for the industrial production of Tirofiban hydrochloride, a medicament for treating angina.
Owner:LUNAN PHARMA GROUP CORPORATION
Tirofiban hydrochloride lyophilized powder injection and preparation method thereof
ActiveCN101756915AGood resolubilityExcellent indicatorsPowder deliveryOrganic active ingredientsWestern medicineTirofiban Hydrochloride
The invention relates to a tirofiban hydrochloride lyophilized powder injection and a preparation method thereof, belonging to the field of western medicine preparation. The tirofiban hydrochloride lyophilized powder injection contains 1 part by weight of tirofiban hydrochloride and 0.5-100 parts by weight of dextran, and the pH value of solution before lyophilization is 1.8-2.6. Preferably, the weight ratio between the dextran and the tirofiban hydrochloride is 2:1-20:1. The tirofiban hydrochloride lyophilized powder injection has good composite dissolubility and stability as well as high safety.
Owner:鲁南新时代生物技术有限公司
A kind of preparation method of tirofiban hydrochloride
ActiveCN102267937AImprove securitySalt forming conditions are simpleOrganic chemistrySolubilityOrganic solvent
The invention provides a preparation method of tirofiban hydrochloride. The preparation method has the advantages of simple salt forming conditions, low carbon and environmentally friendly characteristics, high product purity and no residue of organic solvents. The preparation method is characterized in that tirofiban is dissolved in 0.5 to 1.5 mol / L of a hydrochloric acid solution and based on the character that solubility of a tirofiban hydrochloride product in the hydrochloric acid solution can be influenced greatly by temperature variation, pure tirofiban hydrochloride crystals are obtained though cooling. The pure tirofiban hydrochloride crystals obtained by the preparation method have not only high purity but also no residue of organic solvents. Therefore, clinical medication safetyis improved obviously.
Owner:通辽市华邦药业有限公司
Tirofiban hydrochloride freeze-dried powder injecta and preparing method
ActiveCN1742725ALong validity periodImprove product qualityPowder deliveryOrganic active ingredientsTirofiban HydrochlorideFreeze-drying
The preparation method of tirofiban hydrochloride freeze-dried powder injection preparation includes the following steps: using 30-1200g of lactose, adding water for injection, dissolving, adding active carbon, stirring for 10-60 min at 25-100deg.C, filtering and removing carbon, adding 5.62g of tirofiban hydrochloride monohydrate, stirring, adding water for injection to 1500-4000ml, regulating pH value of solution to 2-3, sterile filtering, filling, freeze-drying, squeezing cap and packaging, etc.
Owner:SHENYANG XINMA PHARMA
Injection containing tirofiban hydrochloride
ActiveCN101716138AReduce the amount of infusionEasy to carryOrganic active ingredientsPharmaceutical delivery mechanismTirofiban HydrochlorideTransfusion volume
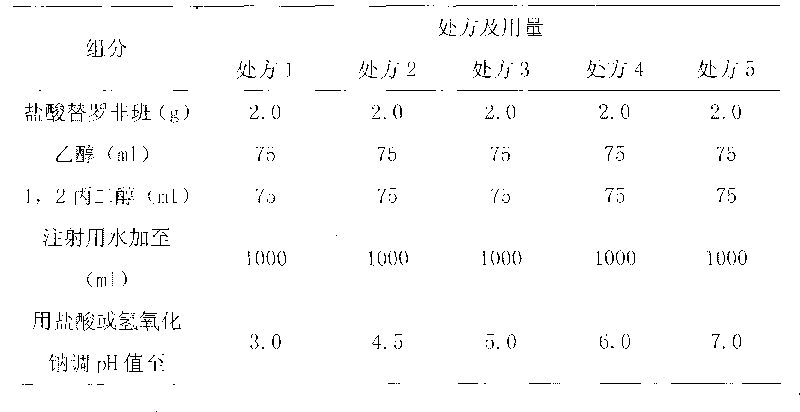
The invention relates to injection containing tirofiban hydrochloride and belongs to the field of pharmaceutical preparations. The injection consists of 0.05 to 0.5 percent (w / v) of tirofiban hydrochloride, 5 to 25 percent (v / v) of 1,2-propylene glycol, 5 to 25 percent(v / v) of ethanol and water, and the pH value of the injection is 3.5 to 6.5. When the injection containing the tirofiban hydrochloride is clinically used, the transfusion volume of a patient is lowered, and the injection can also be added in sodium chloride or glucose transfusion for intravenous drip of the patient, so that the patients suitable to be treated by the injection is increased, and the injection is suitable for more patients suffering from acute coronary syndromes.
Owner:LUNAN PHARMA GROUP CORPORATION
Tirofiban hydrochloride injecta and preparation method thereof
InactiveCN102600072ALess insoluble particlesOrganic active ingredientsPharmaceutical delivery mechanismAspirinTirofiban Hydrochloride
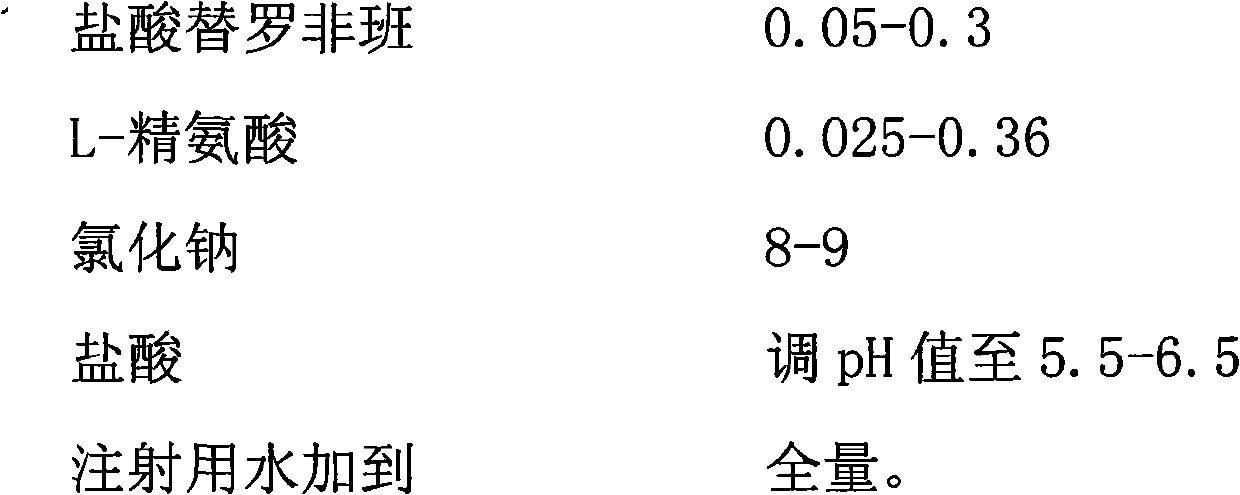
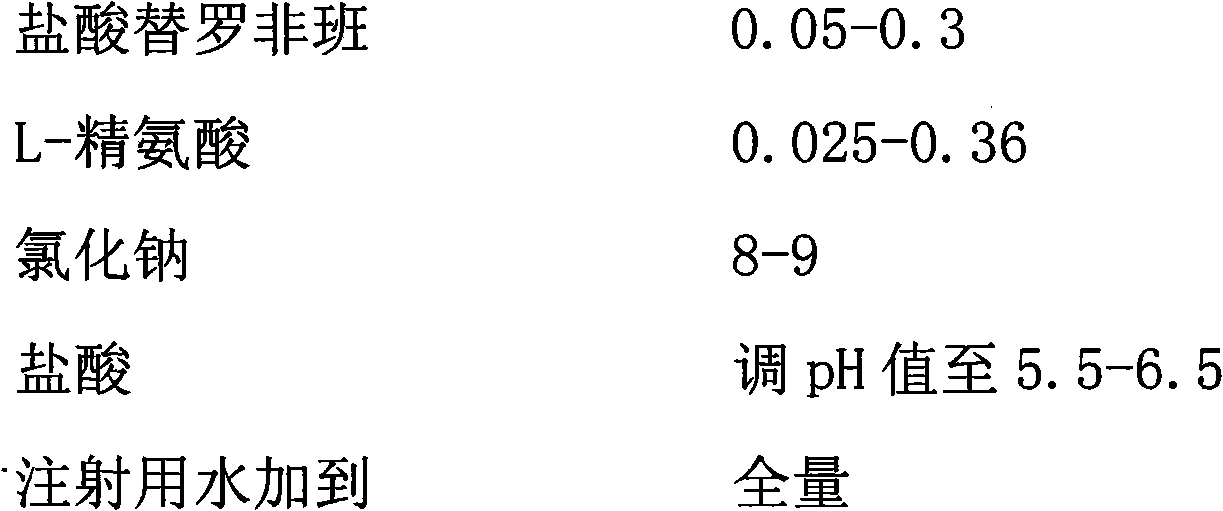
The invention provides a tirofiban hydrochloride injecta and a preparation method thereof. L-arginine is added into an injecta formula, and compared with a formula in which a pH (Potential Of Hydrogen) buffer solution is used as an accessory and a formula provided with arginine aspirin, insoluble particles of the tirofiban hydrochloride injecta are reduced in the formula with the L-arginine, and particularly, after the standing of the solution, the quantity of the insoluble particles is basically not increased as time goes on, so that the tirofiban hydrochloride injecta is ensured to be safe and effective.
Owner:武汉同源药业有限公司
Preparation method of compound tirofiban hydrochloride
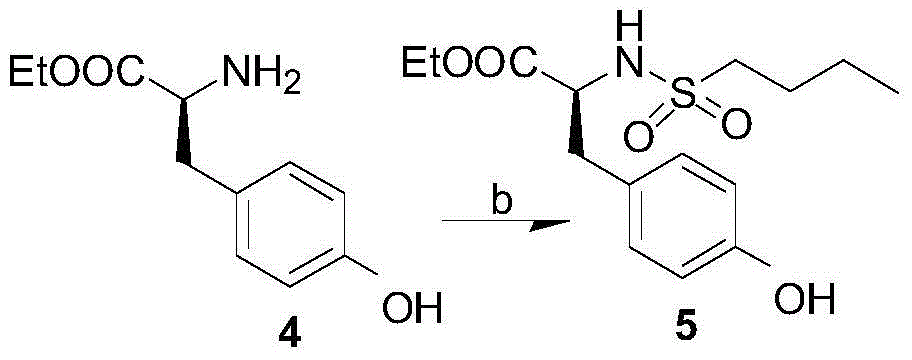
The invention provides a novel synthesis method of tirofiban hydrochloride. The tirofiban hydrochloride is prepared directly through stewing N-butyl sulfonyl-O-4-(4'-pyridyl)-butyl-L-tyrosine in one reactor. The solvent used in the reaction adopts mixture of one of methanol, ethanol, propanol and isopropanol and water. The method overcomes the defects of complex process, high solvent consumption, long production time and easy product isomerization in the original synthesis method, and facilitates the large-scale production.
Owner:武汉同源药业有限公司
Injection use-powder ampoule for inhibiting thrombocyte agglutination and its preparation method
ActiveCN1686127AImprove stabilityDefinite curative effectPowder deliveryBlood disorderFreeze-dryingAgglutination
A powder injection for suppressing the thrombocyte coagulation is proportionally prepared from tirofiban hydrochloride, the water for injection and the pharmacologically acceptable carrier chosen from lactose, mannitol, cane sugar, glucose, dextran, hydrolytic gelatin and sodium chloride through proportionally mixing, examining intermediate, adding water, filtering, loading in bottle and vacuum freeze drying.
Owner:GRAND PHARM (CHINA) CO LTD
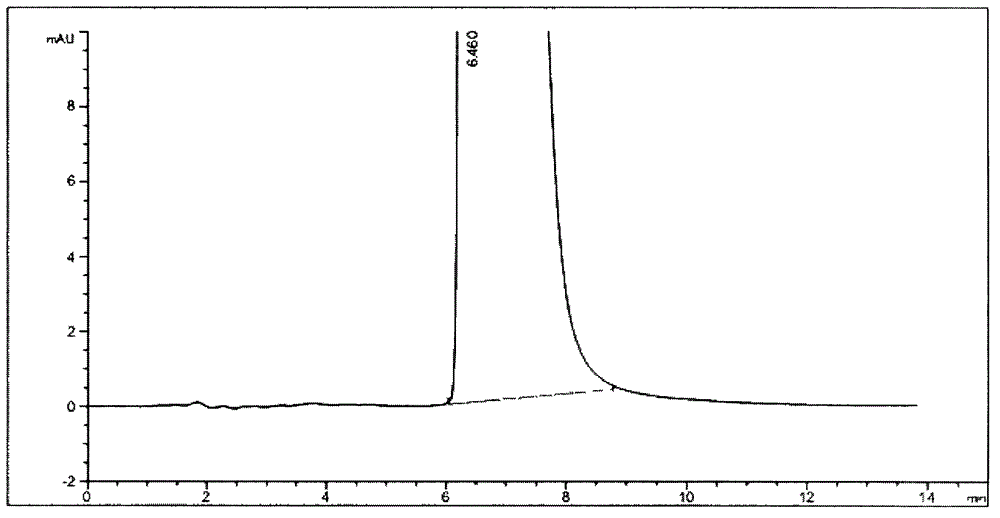
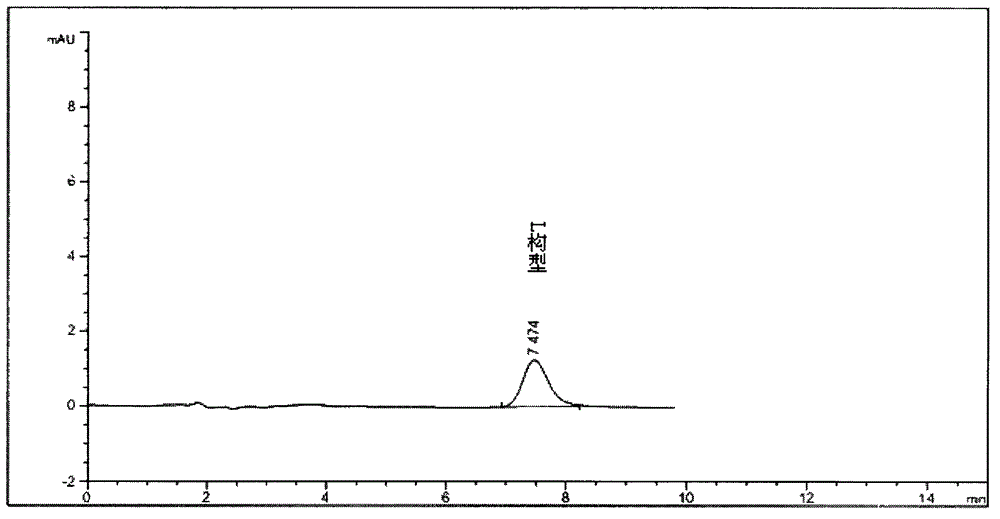
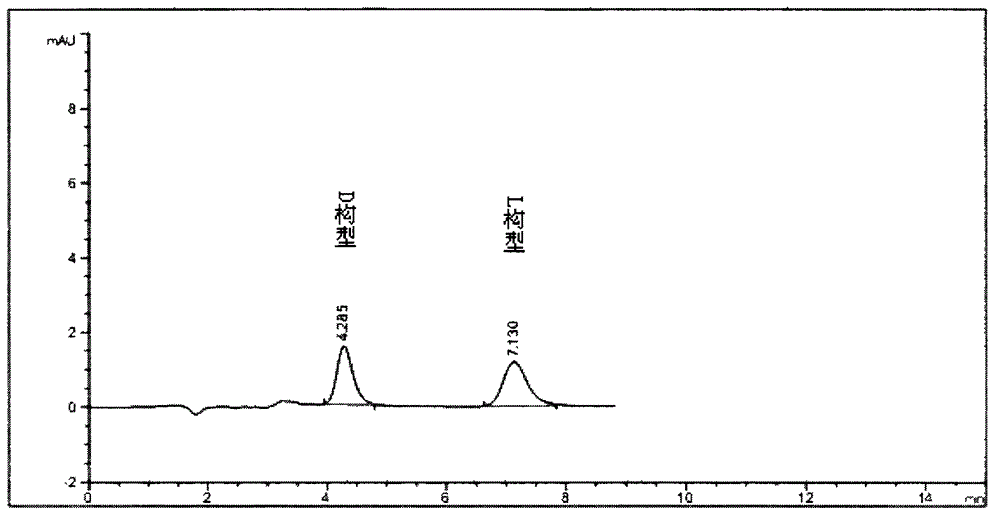
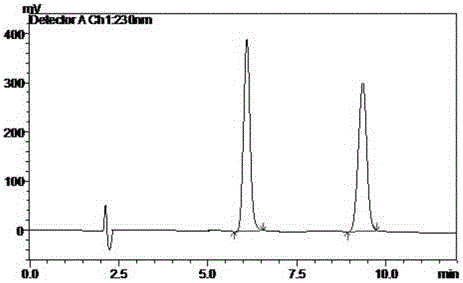
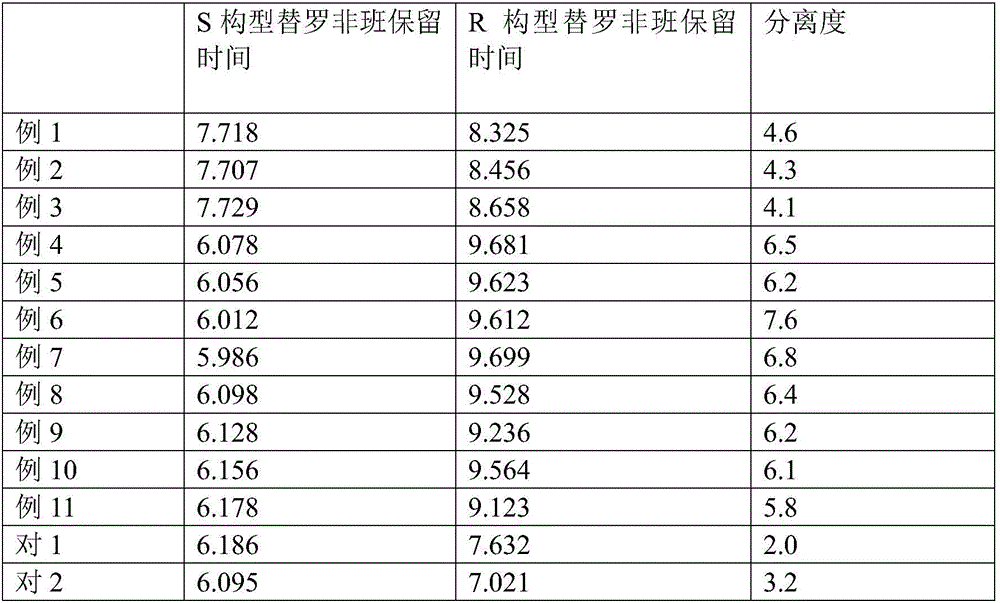
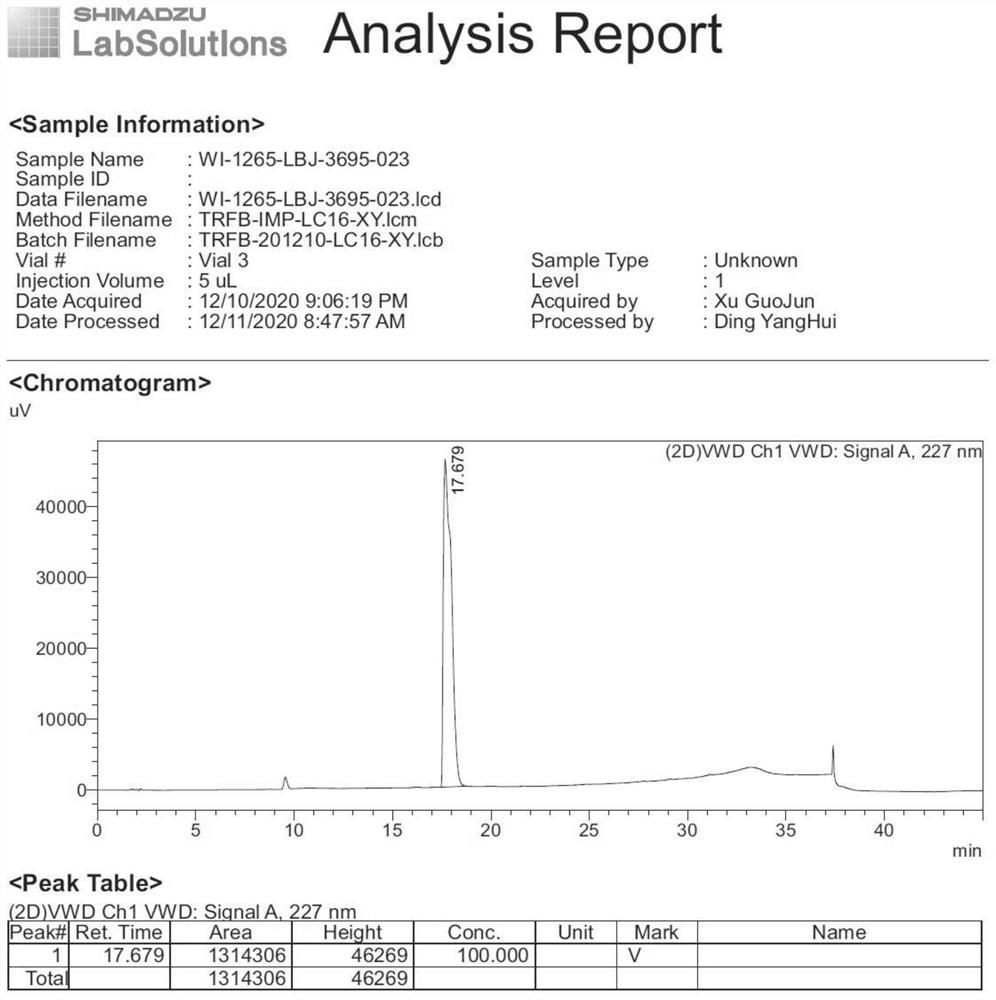
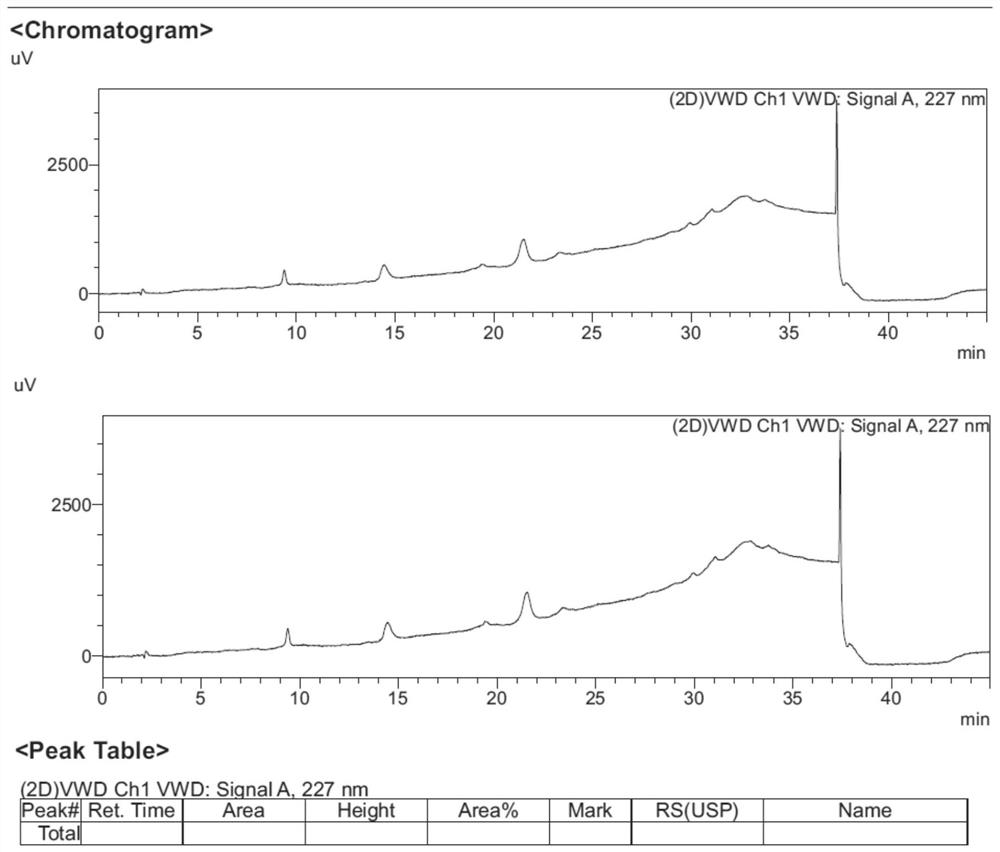
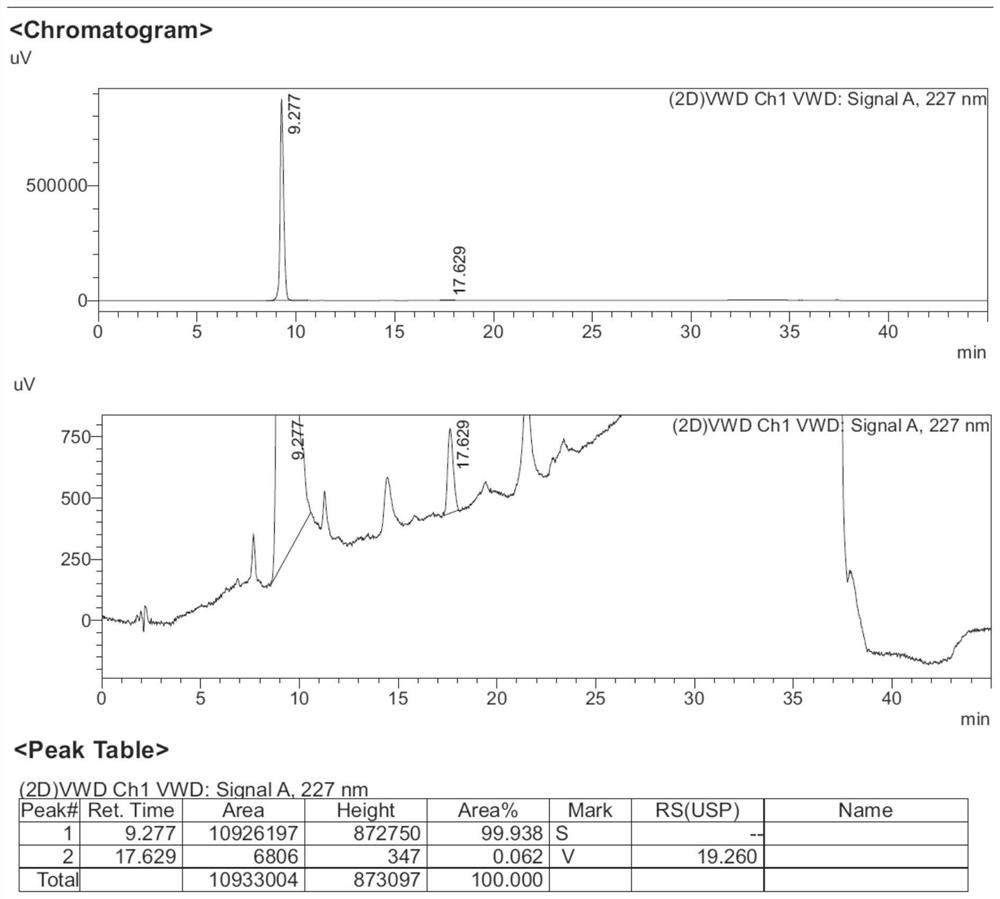
Separation method for tirofiban hydrochloride isomer, and metering method for D-configuration tirofiban hydrochloride
ActiveCN102875449AImprove accuracyOrganic chemistryComponent separationTirofiban HydrochlorideHigh-performance liquid chromatography
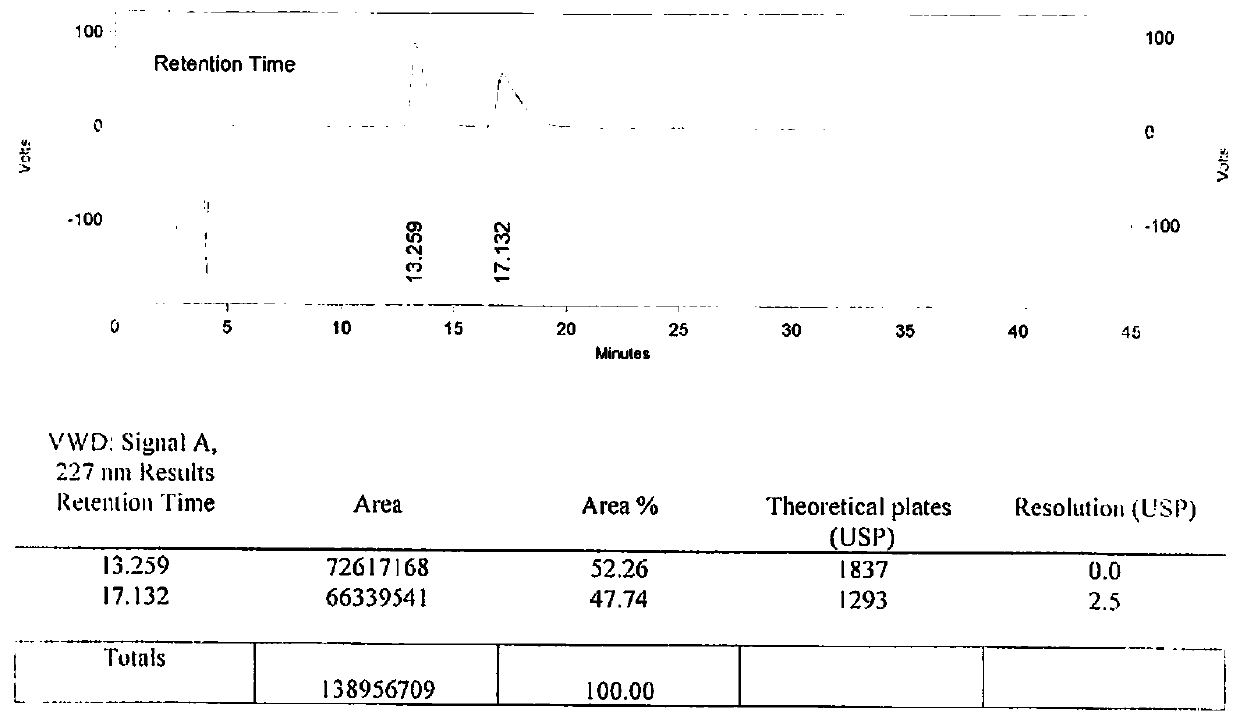
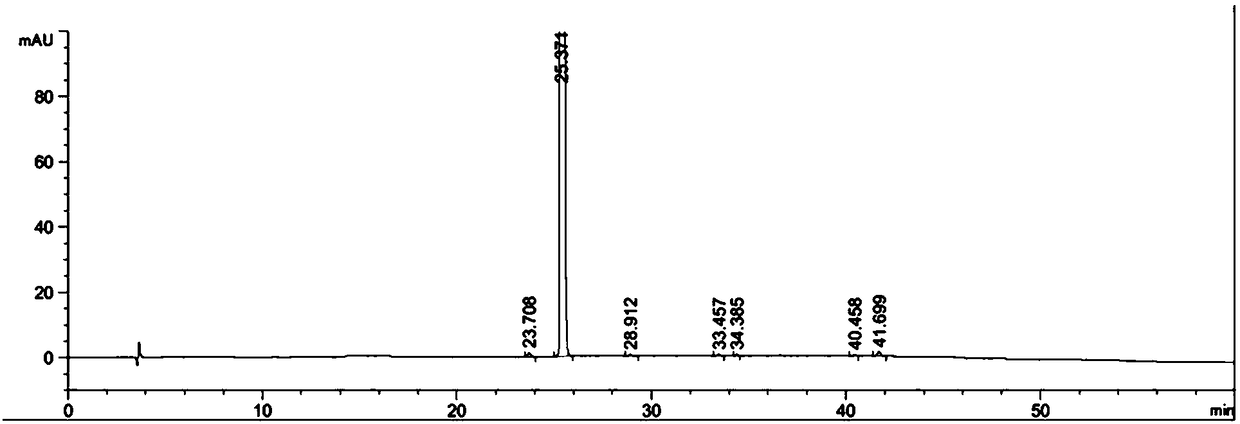
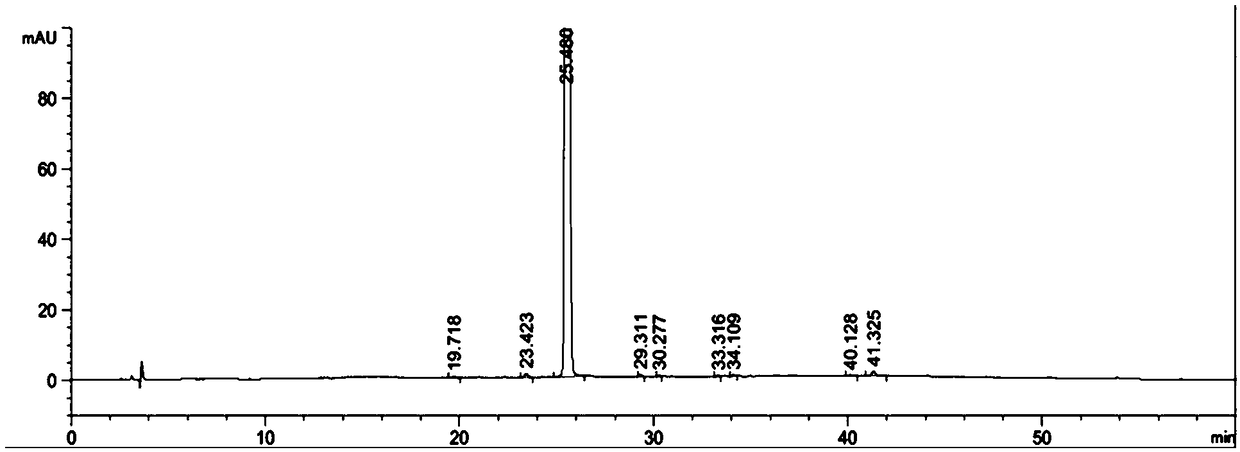
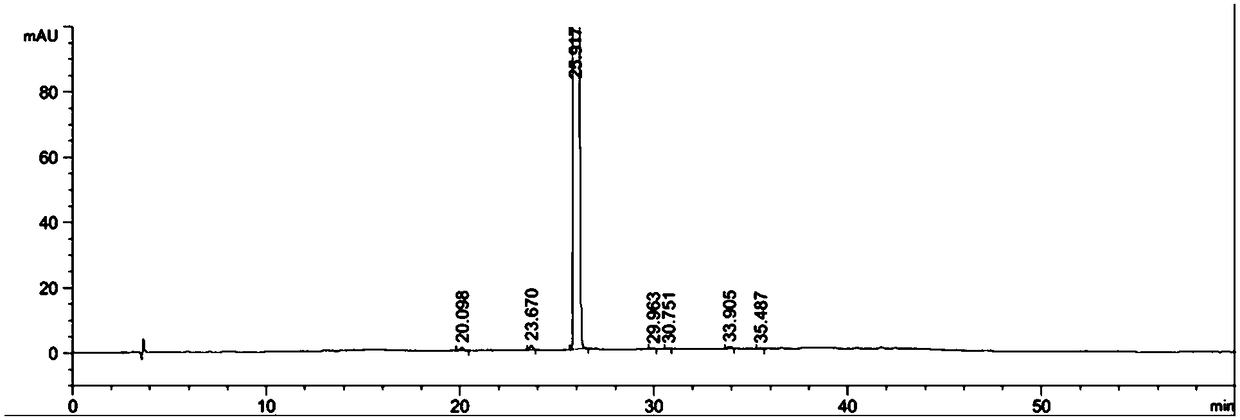
The invention provides a separation method for tirofiban hydrochloride isomer, and a metering method for D-configuration tirofiban hydrochloride. By adjusting high performance liquid chromatography conditions, the separation flexibility of the tirofiban hydrochloride isomer is improved by 3 to 10 times, and the separation degree is improved to be more than 2.0, so that the accuracy of the D-configuration tirofiban hydrochloride is improved obviously.
Owner:通辽市华邦药业有限公司
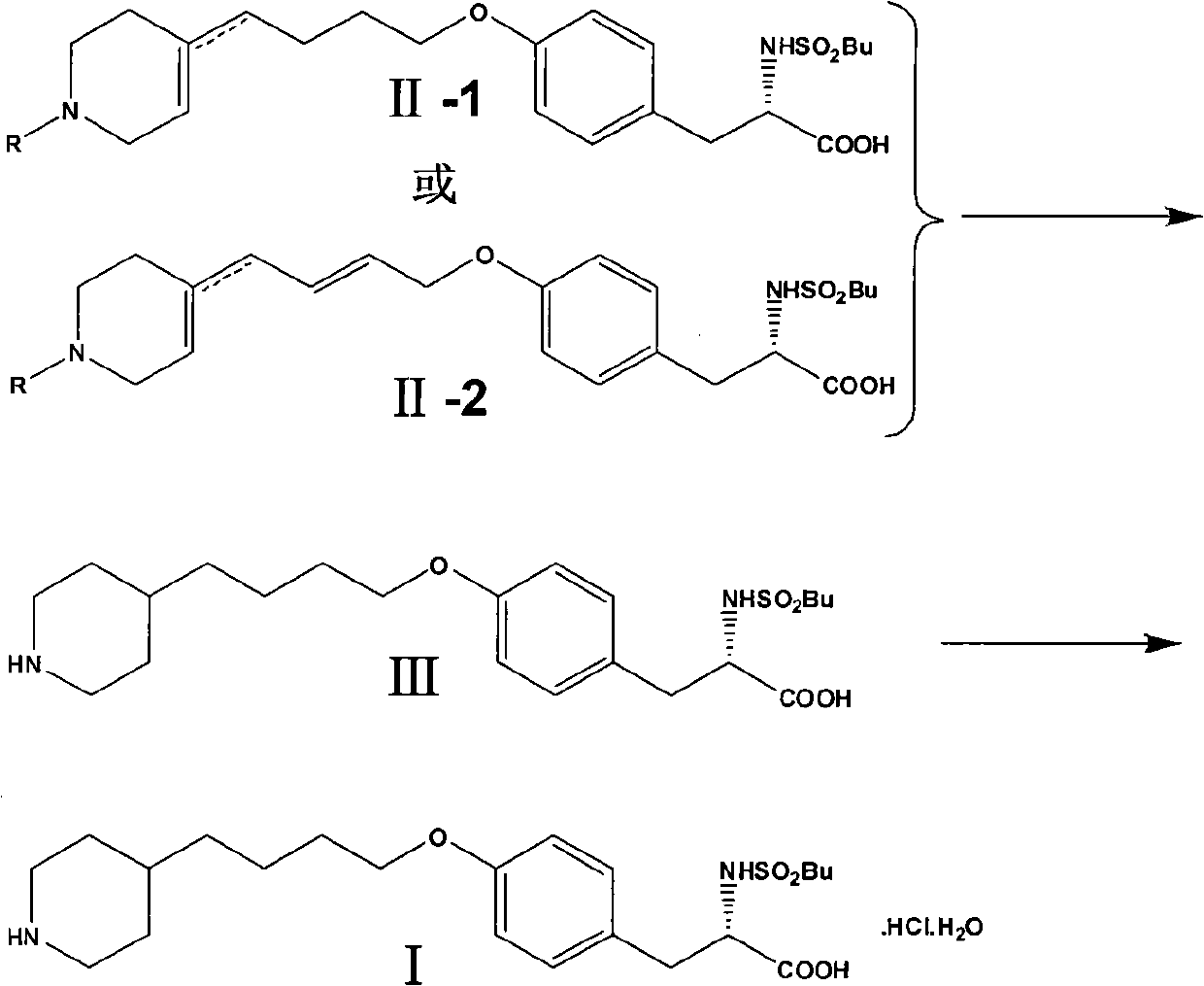
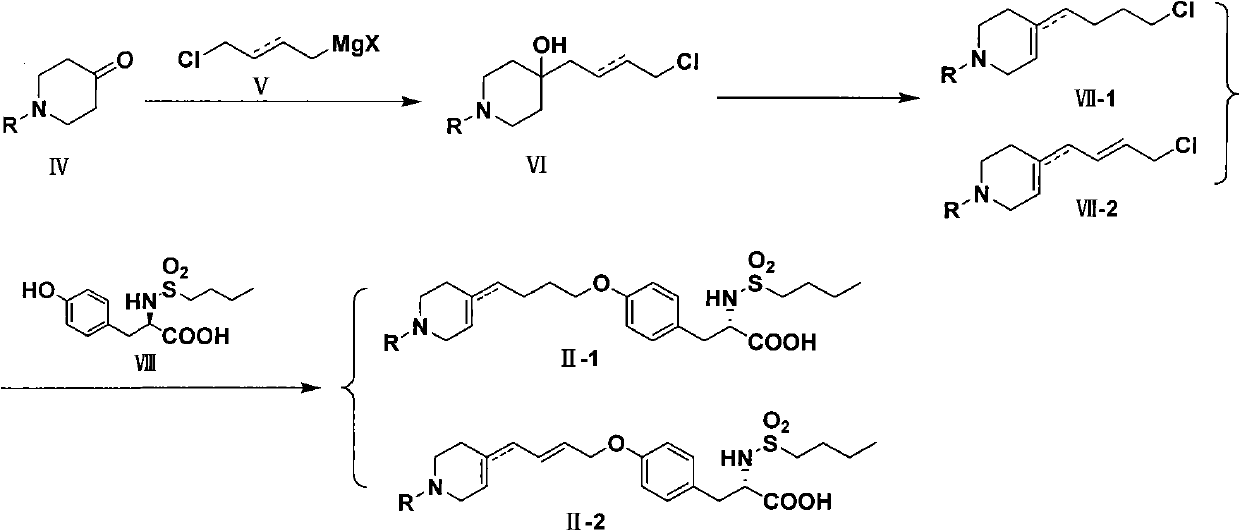
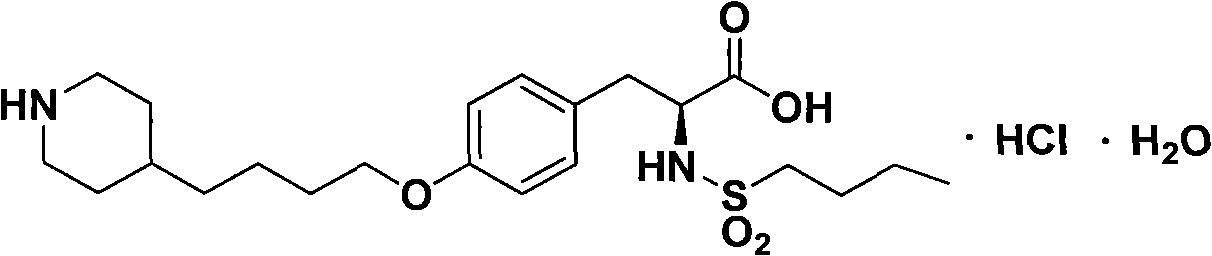
Method for preparing tirofiban hydrochloride
InactiveCN102241622ASafe preparationMild reaction conditionsOrganic chemistryTirofiban HydrochlorideMedicinal chemistry
The invention discloses a method for preparing tirofiban hydrochloride. The method comprises the following steps: reducing a compound represented by a formula II-1 or a compound represented by a formula II-2 to obtain tirofiban represented by a formula III; carrying out salt formation for the tirofiban to transform into the target compound tirofiban hydrochloride (I). The method provided by the present invention has advantages of safe preparation process, mild reaction conditions, few steps, easy operation, high yield, cheap and available raw materials, easy treatment of three waste and convenience of industrial implementation. The reaction formulas for preparing the tirofiban hydrochloride are as follows.
Owner:SHANGHAI INST OF PHARMA IND CO LTD
Tirofiban hydrochloride freeze-dried powder injection preparation and preparation method thereof
ActiveCN101716148AImprove solubilitySimple preparation processPowder deliveryOrganic active ingredientsForeign matterSolubility
The invention relates to a tirofiban hydrochloride freeze-dried powder injection preparation and a preparation method thereof. The tirofiban hydrochloride freeze-dried powder injection preparation produced by a freeze-dried technology of the invention greatly enhances solubility of tirofiban hydrochloride; and since the pH of redissolved liquid medicine is within the range of 2.0-8.0 and visible particles and particulate matter satisfy the requirement of the pharmacopeia, the safety of medicine is greatly improved. The tirofiban hydrochloride freeze-dried powder injection preparation has simple preparation, controllable quality, stable physical and chemical properties and safe and effective use.
Owner:LUNAN PHARMA GROUP CORPORATION
Tirofiban hydrochloride preparation process
ActiveCN104447509AProcess reaction conditions are mildHigh yieldOrganic chemistryChemical industryTirofiban Hydrochloride
The invention belongs to the field of medicine and chemical industry. According to the present invention, the tirofiban hydrochloride prepared through substitution, condensation, reduction, salification and other reactions has characteristics of high yield, good quality and good stability, and the preparation process of the present invention provides a synthesis process suitable for the practical industrial mass production.
Owner:HARVEST PHARMA HUNAN CO LTD
Tirofiban hydrochloride lyophilization powder injection
ActiveCN102423303AAdvantages and Notable ImprovementsImprove solubilityOrganic active ingredientsPowder deliveryForeign matterTirofiban Hydrochloride
The invention relates to a tirofiban hydrochloride lyophilization powder injection. A lyophilization excipient which is used in the invention is cysteine hydrochloride, and the weight ratio of tirofiban hydrochloride (by free alkali) to cysteine hydrochloride is 1:0.25-8, preferably 1:0.5-4, and most preferably 1:1-2. The tirofiban hydrochloride lyophilization powder injection of the invention, which has the advantages of stable physical and chemical property and controllable quality, solves an unqualified phenomenon that there are foreign matters when tirofiban hydrochloride is redissolved.
Owner:SHANDONG NEWTIME PHARMA
Injectio for inhibiting platelet aggregation and its preparation process
ActiveCN1322863CImprove stabilityDefinite curative effectOrganic active ingredientsPharmaceutical delivery mechanismActivated carbonTirofiban Hydrochloride
The invention relates to an injection for inhibiting platelet aggregation and its preparation process, wherein the injection comprises Tirofiban Hydrochloride, water and sodium chloride, and is prepared through the steps of, weighing sodium chloride by formula amount, charging into 5000ml water, stirring to complete dissolving, weighing activated charcoal 0.3% of the solution amount, and homogenizing, heating and boiling for 15 minutes, cooling down, filtering the activated charcoal, accurately weighing Tirofiban Hydrochloride by formula amount, dissolving completely with water, charging into the sodium chloride solution, and watering to near total amount, adjusting the pH to 5.5-6.5 with hydrochloric acid, watering to specified amount, filtering with 0.45 um microporous filtration film, split charging into bottle, sterilizing 35 minutes through hot compression at 115 deg. C.
Owner:GRAND PHARM (CHINA) CO LTD
Tirofiban hydrochloride freeze-dried powder injecta and preparing method
ActiveCN100367961CLong validity periodImprove product qualityPowder deliveryOrganic active ingredientsTirofiban HydrochlorideFreeze-drying
The preparation method of tirofiban hydrochloride freeze-dried powder injection preparation includes the following steps: using 30-1200g of lactose, adding water for injection, dissolving, adding active carbon, stirring for 10-60 min at 25-100deg.C, filtering and removing carbon, adding 5.62g of tirofiban hydrochloride monohydrate, stirring, adding water for injection to 1500-4000ml, regulating pH value of solution to 2-3, sterile filtering, filling, freeze-drying, squeezing cap and packaging, etc.
Owner:SHENYANG XINMA PHARMA
Palladium removal method for preparation process of tirofiban hydrochloride
InactiveCN109336807AAvoid residueGuaranteed palladium removal effectOrganic chemistryArylOrganic solvent
The invention provides a palladium removal method for a preparation process of tirofiban hydrochloride. The method comprises the following steps: N-(butylsulfonyl)-O-[4-(4-piperidynyl)butyl]-L-tyrosine (tirofiban) is added to an organic solution of trialkyl(aryl)phosphine, and the substances are sufficiently stirred and filtered; a filter cake is washed with an organic solvent and dried, and content of remaining palladium is reduced to 1 ppm or lower. Palladium content is reduced for tirofiban hydrochloride prepared with the palladium removal method for the preparation process of tirofiban hydrochloride, and the whole method is simple to operate, has remarkable effect and is applicable to large-scale production.
Owner:无锡富泽药业有限公司
Method for preparing tirofiban hydrochloride intermediate
ActiveCN101898998AHigh purityThe purity is greater than 99.0%, and the total yield is highOrganic chemistryTirofiban HydrochlorideButanol
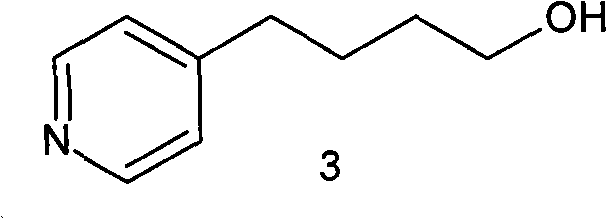
The invention relates to a method for preparing a compound, in particular to a method for preparing 4-(4-pyridyl)-1-butanol which is a tirofiban hydrochloride intermediate. The preparation method has high yield, causes little waste solid, liquid and gas, ensures high product purity and successfully realizes industrial production.
Owner:WUHAN WUYAO SCI & TECH
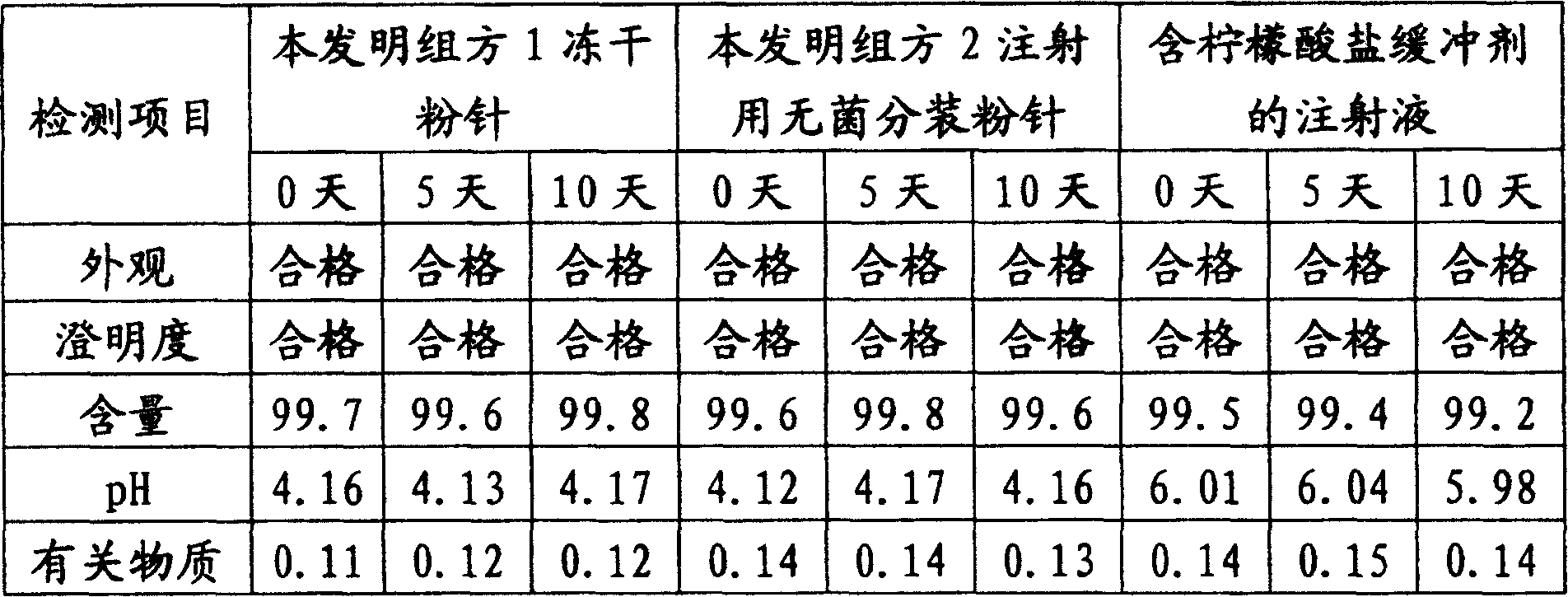
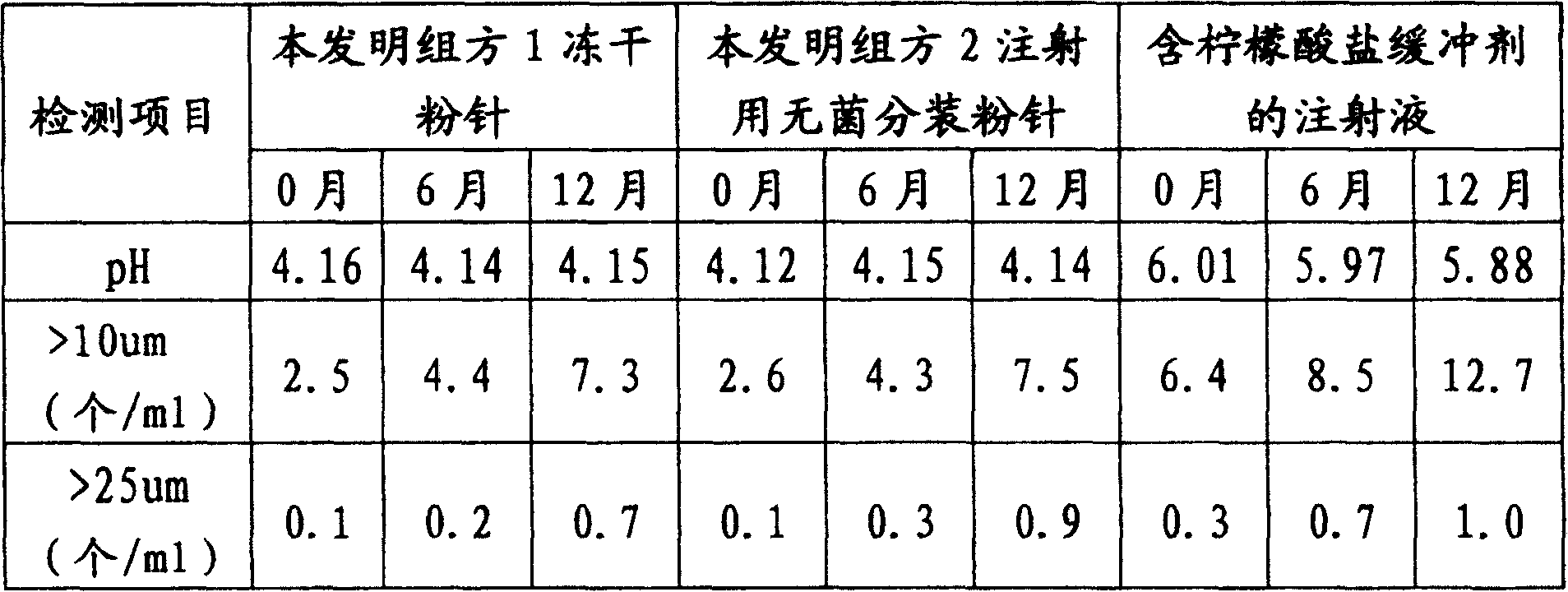
Tirofiban hydrochloride injection and preparation method thereof
ActiveCN108743527AProgressiveHigh content of active substancesOrganic active ingredientsPharmaceutical delivery mechanismTirofiban HydrochlorideSodium citrate
The invention relates to tirofiban hydrochloride injection and belongs to the field of pharmaceutical preparation. The injection disclosed by the invention is prepared from tirofiban hydrochloride, sodium alginate, a pH regulator and water. A preparation method comprises the steps of dissolving citric acid and sodium citrate into 10 to 50% of water to be prepared into a citric acid-sodium citratebuffer solution, then dissolving the tirofiban hydrochloride and the sodium alginate into water and mixing two solutions; adjusting a pH value and then filtering, subpackaging, disinfecting, light checking and packaging to obtain the tirofiban hydrochloride injection. According to the tirofiban hydrochloride injection disclosed by the invention, an osmotic pressure regulator is optimally chosen, acontent of active substances is high, and stability of a main drug can be kept under the conditions of high temperature, illumination and the like; a preparation technology has the advantages of simpleness, suitability for industrial production and convenience in market popularization.
Owner:LUNAN PHARMA GROUP CORPORATION
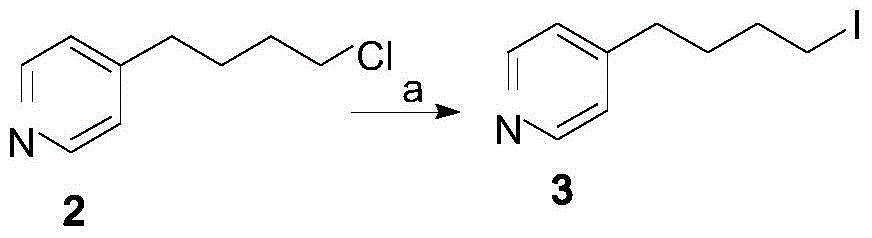
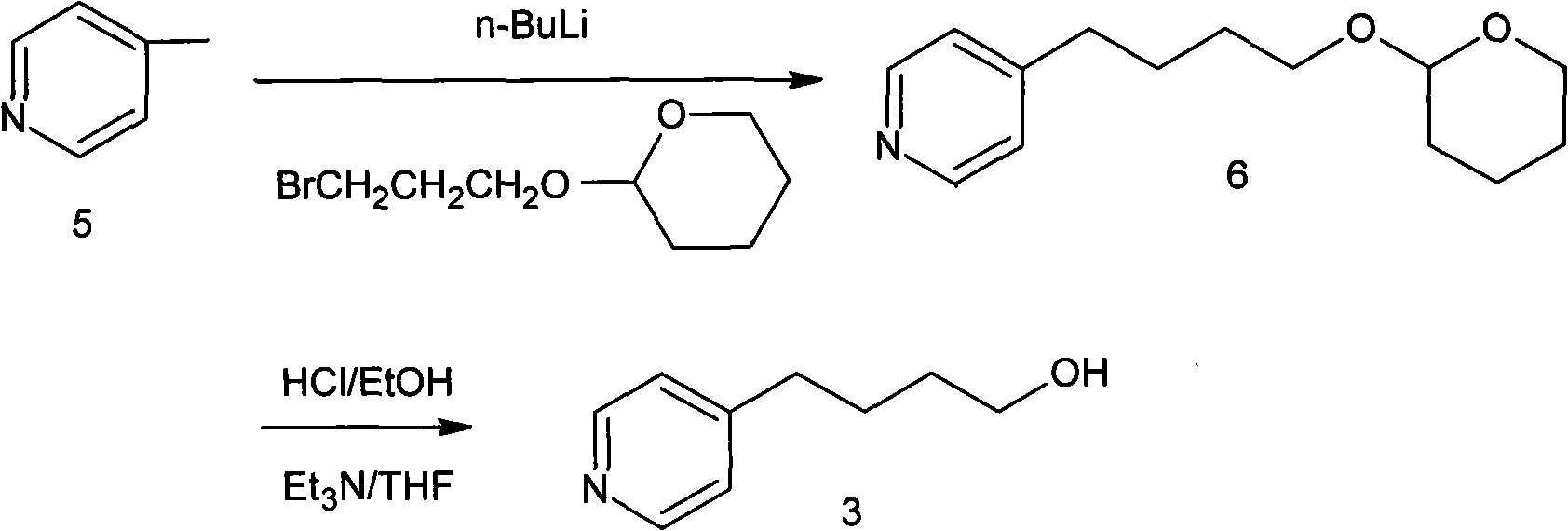
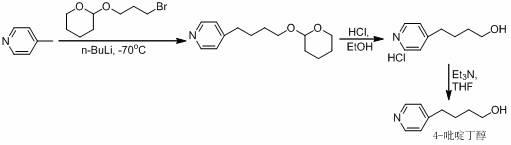
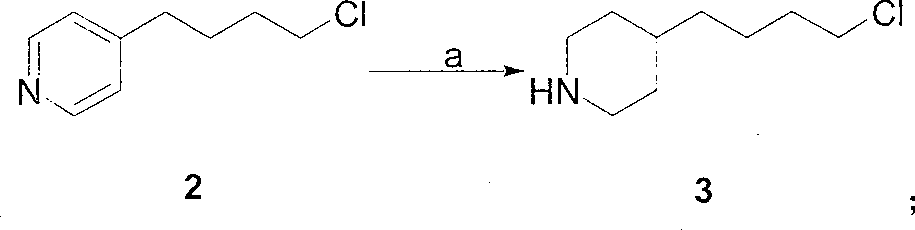
A New Process for the Preparation of 4-Pyridine Butanol
The invention relates to a new preparation process of tirofiban hydrochloride key intermediate 4-pyridine butanol. The key to the process is to make the reaction intermediate 4-(4-pyridyl)-3-butyn-1-ol into hydrochloride or sulfate to improve its stability, and the corresponding salt can be hydrogenated under normal temperature and pressure to obtain the corresponding Pyridine butanol hydrochloride or sulfate, the latter can prepare high-purity 4-pyridine butanol by distillation under reduced pressure after freeing triethylamine. This route does not involve expensive reagents, has low requirements for equipment, is simple to operate, and is highly feasible for industrial scale-up.
Owner:WISDOM PHARM CO LTD
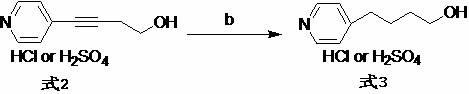
Method for separating and detecting cardio-cerebrovascular drug S-configuration tirofiban
A method for separating and detecting a cardio-cerebrovascular drug S-configuration tirofiban includes the steps of (1), weighing tirofiban hydrochloride racemate and subjecting the tirofiban hydrochloride racemate to extraction by a flowing-phase solution so as to obtain an injection sample; (2), adopting high-performance liquid chromatography for chiral separation. The method for separating and detecting the cardio-cerebrovascular drug S-configuration tirofiban has the advantages that the method is excellent in separation degree and capable of reducing chiral separation cost greatly because of cheap and easily available chitosan, thereby being suitable for large-scale industrial production.
Owner:上海微谱检测科技集团股份有限公司 +1
Tirofiban hydrochloride lyophilized powder injection and preparation method thereof
ActiveCN101756915BGood resolubilityExcellent indicatorsPowder deliveryOrganic active ingredientsWestern medicineTirofiban Hydrochloride
The invention relates to a tirofiban hydrochloride lyophilized powder injection and a preparation method thereof, belonging to the field of western medicine preparation. The tirofiban hydrochloride lyophilized powder injection contains 1 part by weight of tirofiban hydrochloride and 0.5-100 parts by weight of dextran, and the pH value of solution before lyophilization is 1.8-2.6. Preferably, the weight ratio between the dextran and the tirofiban hydrochloride is 2:1-20:1. The tirofiban hydrochloride lyophilized powder injection has good composite dissolubility and stability as well as high safety.
Owner:鲁南新时代生物技术有限公司
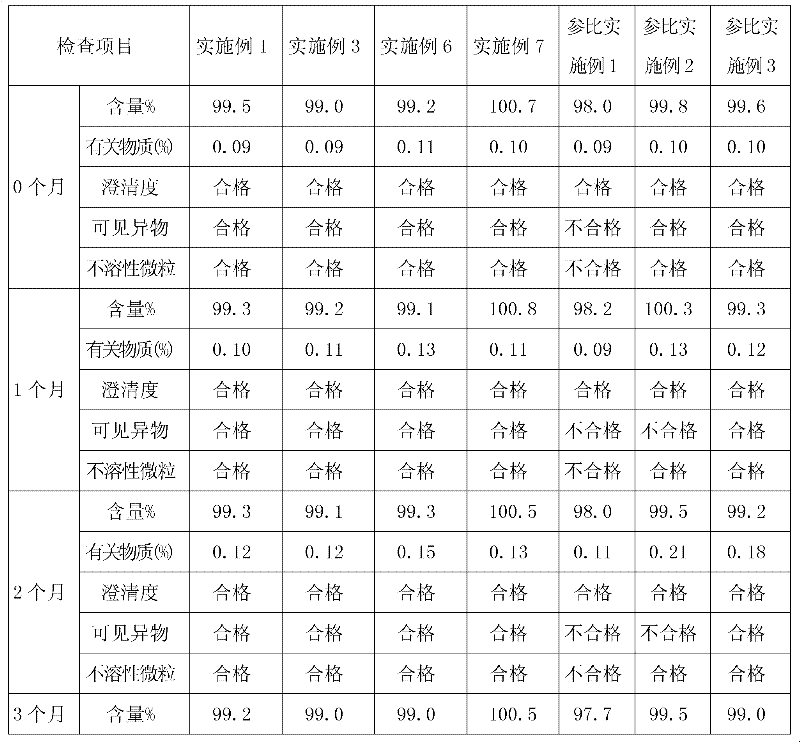
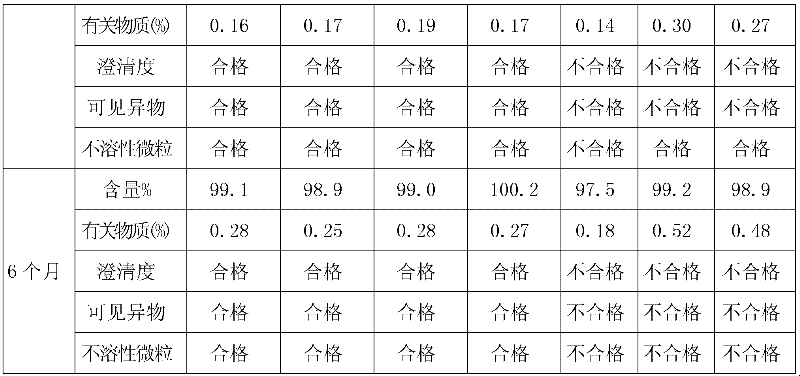
Tirofiban hydrochloride intermediate and preparation method of tirofiban hydrochloride
ActiveCN111100066AReduce contentComply with medicinal requirementsOrganic active ingredientsComponent separationUse medicationClinical efficacy
The invention provides a tirofiban hydrochloride intermediate and a preparation method of tirofiban hydrochloride. The content of isomers of the tirofiban hydrochloride intermediate and tirofiban hydrochloride prepared by the method is not higher than 0.5%, preferably not higher than 0.3%, and more preferably not higher than 0.1%; all indexes of the prepared pharmaceutical composition meet the medicinal requirements, the quality is stable in the placing process, and the clinical curative effect and the medication safety can be guaranteed.
Owner:SHIJIAZHUANG PHARMA GRP NBP PHARMA CO LTD +1
Process for preparation of tirofiban hydrochloride
ActiveCN100537536CHigh overall process yieldEasy to operate without dangerOrganic chemistryTirofiban HydrochlorideMedicine
The invention discloses a method suitable for industrial production of tirofiban hydrochloride, a medicine for treating angina pectoris.
Owner:LUNAN PHARMA GROUP CORPORATION
Method of preparing tirofiban hydrochloride
InactiveCN103848775ALow requirements for production equipmentMild reaction conditionsOrganic chemistryTirofiban HydrochlorideChemical compound
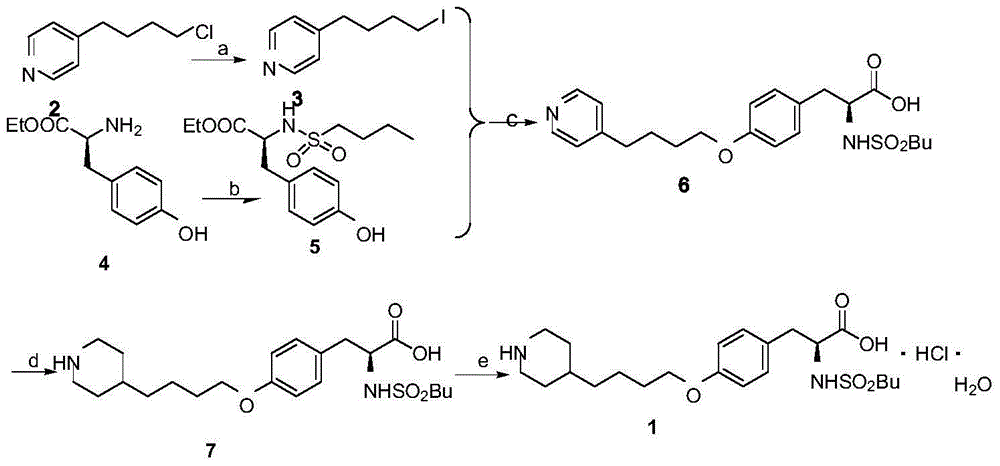
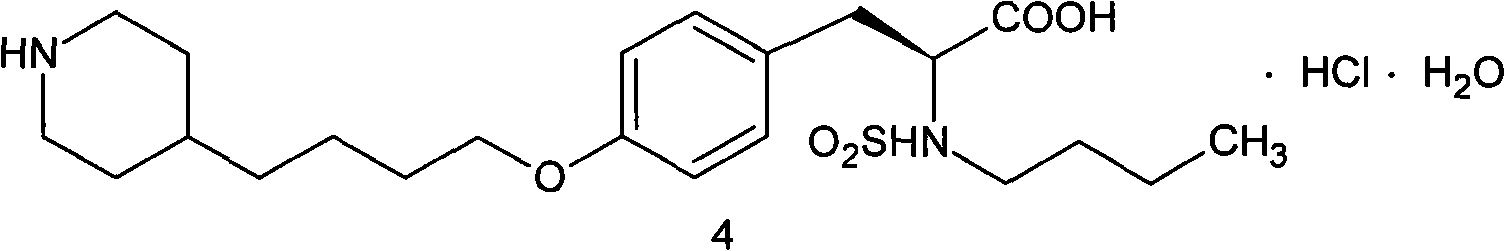
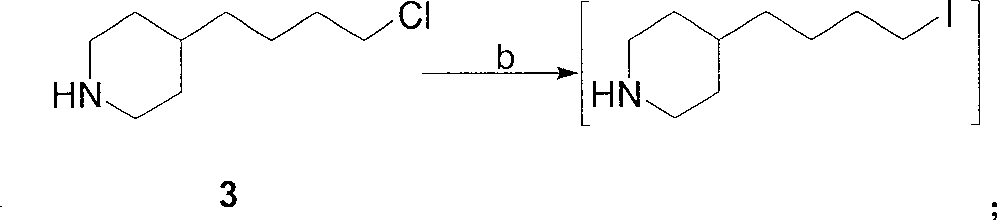
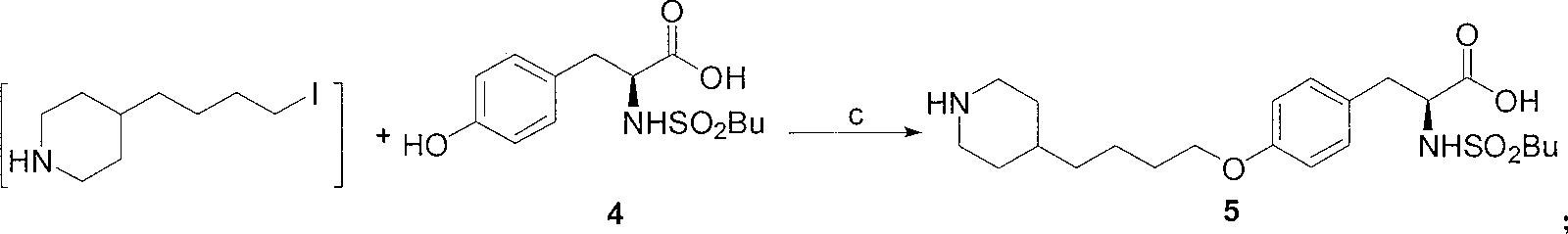
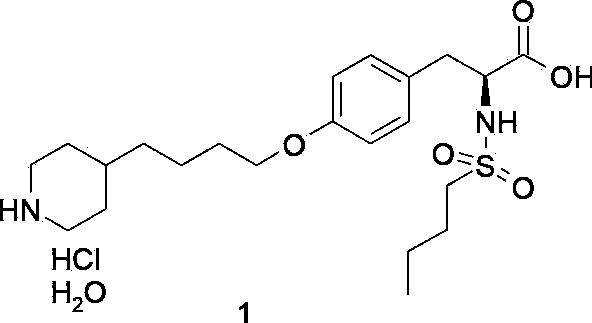
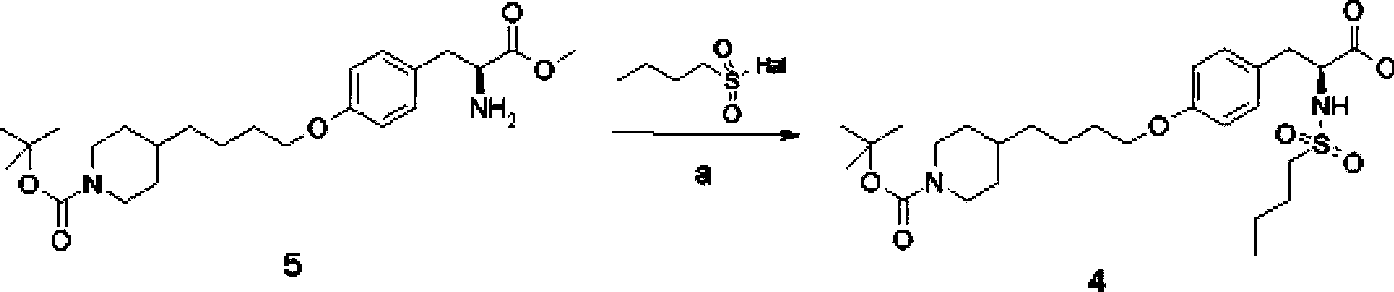
A method of preparing tirofiban hydrochloride is provided. The method includes steps of: (a) reacting a chemical compound 5 with n-butylsulfonyl halide under alkaline conditions to produce a chemical compound 4; (b) removing boc protection of the chemical compound 4 under strong acid conditions to obtain a chemical compound 3; (c) hydrolyzing the chemical compound 3 under alkaline conditions to turn the compound 3 from an ester into an acid so as to obtain a chemical compound 2; and (d) acidizing the chemical compound 2 to obtain the tirofiban hydrochloride 1.
Owner:SHANGHAI SINE PHARMA LAB
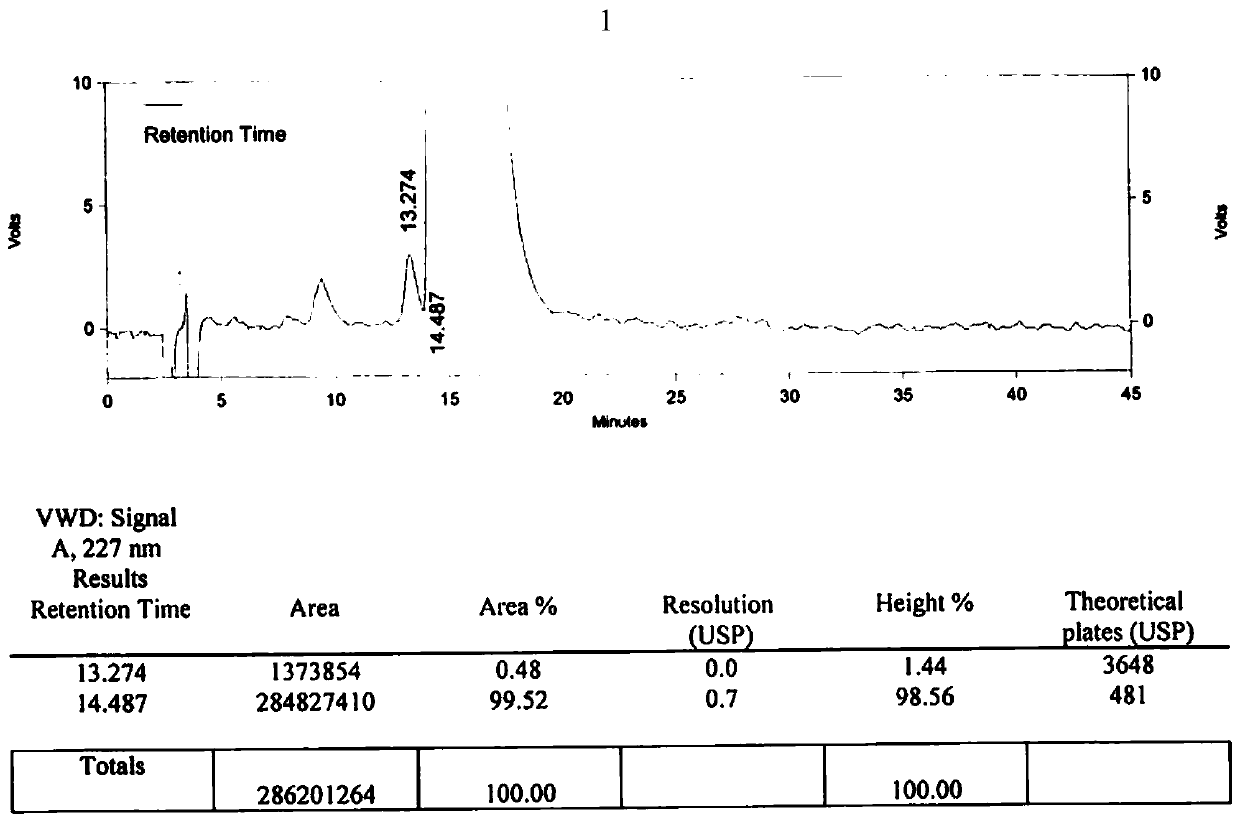
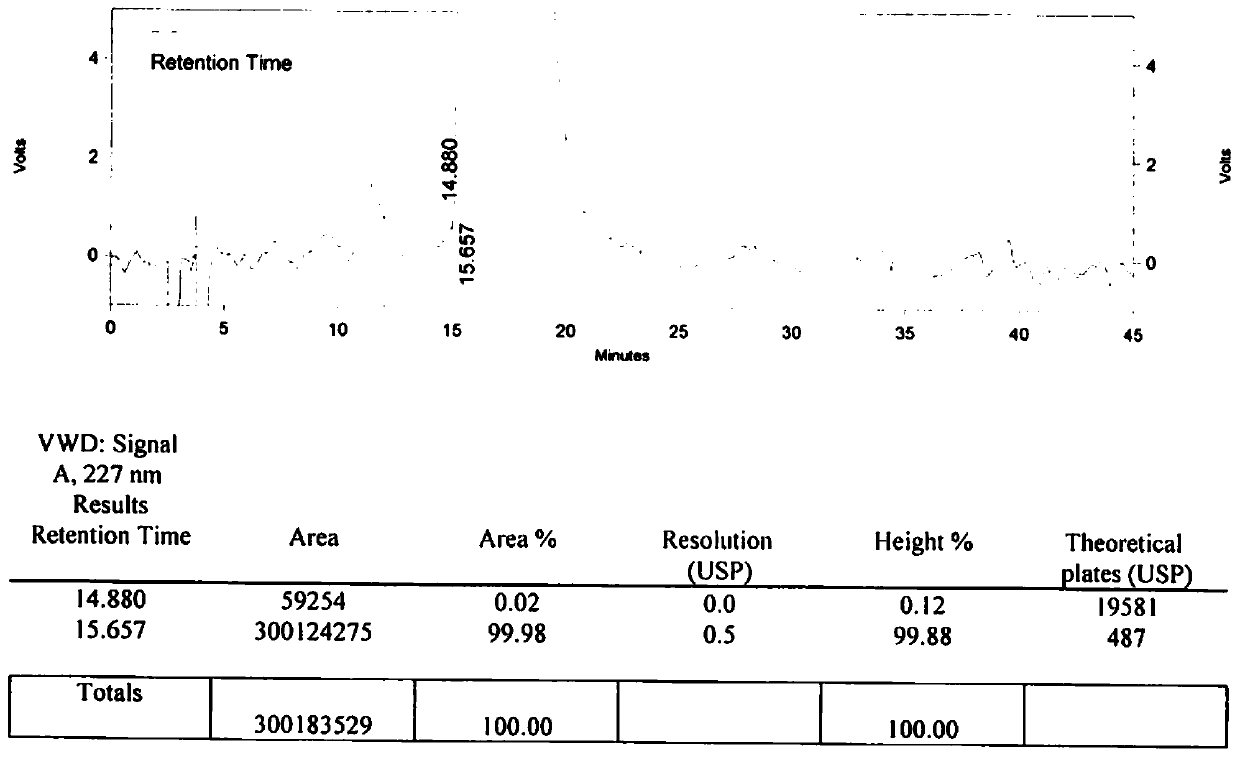
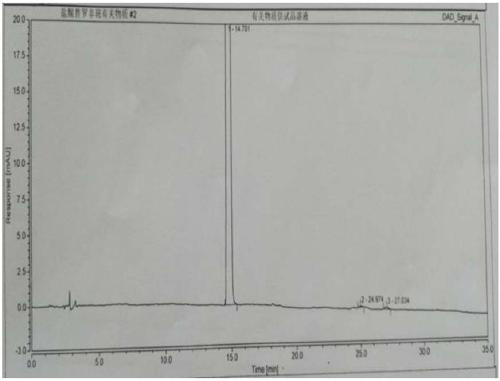
Method for detecting related substances of tirofiban hydrochloride injection
InactiveCN110988158AQuality improvementStrong specificityComponent separationSilica gelPharmaceutical Substances
The invention belongs to the field of drug analysis. The invention relates to a method for detecting related substances of tirofiban hydrochloride injection, in particular to a method for detecting related substances of tirofiban hydrochloride injection by using high performance liquid chromatography, a chromatographic column taking octadecyl bonded silica gel as a filler is used as a stationary phase, a buffer solution with the pH value of 2.0-3.0 is used as a mobile phase A, an organic solvent is used as a mobile phase B, and the detection wavelength is 222-240nm. The detection method can effectively detect the related substances of the tirofiban hydrochloride injection, has strong specificity and high sensitivity, and is suitable for detecting the related substances of the tirofiban hydrochloride injection.
Owner:LUNAN PHARMA GROUP CORPORATION
Drying method for tirofiban hydrochloride
InactiveCN109028765AReduce moisture contentLow impurity contentDrying solid materials with heatDrying gas arrangementsTirofiban HydrochlorideSodium Chloride Injection
The invention provides a drying method for tirofiban hydrochloride. A tirofiban hydrochloride wet product is subjected to fluidized drying through an efficient fluidized drying machine, wherein the drying temperature in the efficient fluidized drying machine is 40-50 DEG C, the drying time is 10-20 minutes, and the air flow is 30-70 m3 / h. The tirofiban hydrochloride prepared through the drying method for the tirofiban hydrochloride is low in water content, is an off-white solid in appearance, meets the requirement for preparing a tirofiban hydrochloride sodium chloride injection, and has the advantages of being not likely to be caked and being low in impurity content.
Owner:无锡富泽药业有限公司
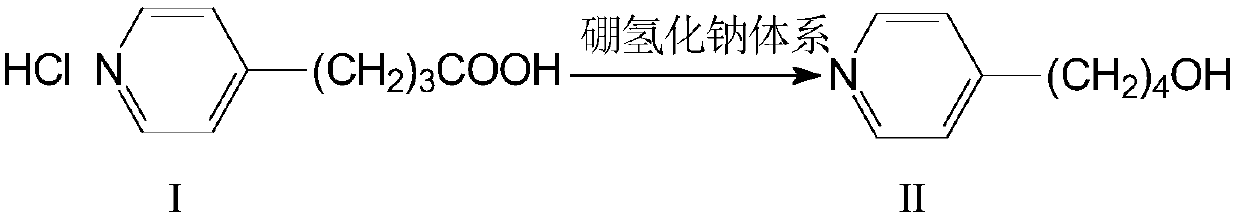
Synthesis method of tirofiban hydrochloride intermediate III
The invention belongs to the field of medicine synthesis, and particularly relates to a synthesis method of a tirofiban hydrochloride intermediate III. The method comprises: under the action of a sodium borohydride reduction system, reducing an intermediate I to obtain an intermediate II, performing a chlorination reaction on the intermediate II and SOCl2 in the presence of Lewis acid chloride toobtain an intermediate III, performing etherification on the intermediate III and N-butyl sulfonyl-L-tyrosine in the presence of an iodination reagent, and performing hydrogenation and salification toobtain tirofiban hydrochloride. The method has the advantages of mild reaction conditions, simple process operation, no need of column chromatography purification in the intermediate steps, reactionyield increase, high product purity, meeting of the use requirements of pharmacopeia standard bulk drugs in injections, substantial improvement of the safety of clinical medication, and suitableness for industrial production.
Owner:LUNAN PHARMA GROUP CORPORATION
Injection use-powder ampoule for inhibiting thrombocyte agglutination and its preparation method
A powder injection for suppressing the thrombocyte coagulation is proportionally prepared from tirofiban hydrochloride, the water for injection and the pharmacologically acceptable carrier chosen from lactose, mannitol, cane sugar, glucose, dextran, hydrolytic gelatin and sodium chloride through proportionally mixing, examining intermediate, adding water, filtering, loading in bottle and vacuum freeze drying.
Owner:GRAND PHARM (CHINA) CO LTD
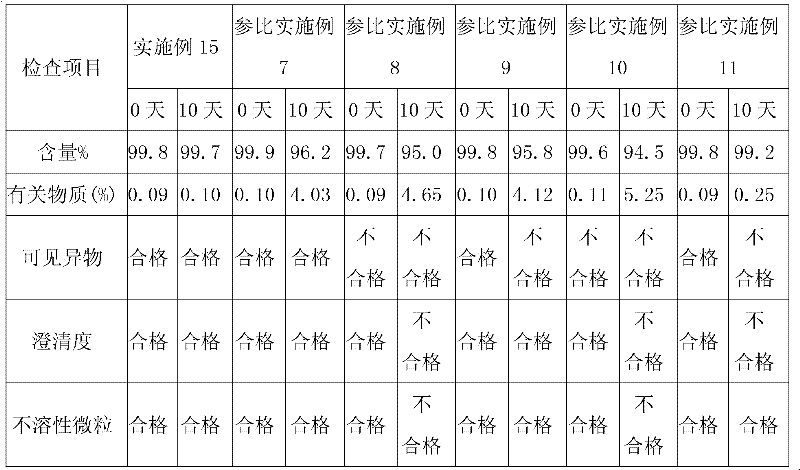
Related substance of tirofiban hydrochloride as well as preparation and detection method of related substance
ActiveCN112816282AHigh purityComponent separationPreparing sample for investigationDrug utilisationTirofiban Hydrochloride
The invention provides a method for preparing related substances of raw material medicine tirofiban hydrochloride. The related substance is never reported by other literature documents. The synthesis and research of the related substance are beneficial to improving the quality and medication safety of the bulk drug tirofiban hydrochloride.
Owner:WISDOM PHARM CO LTD
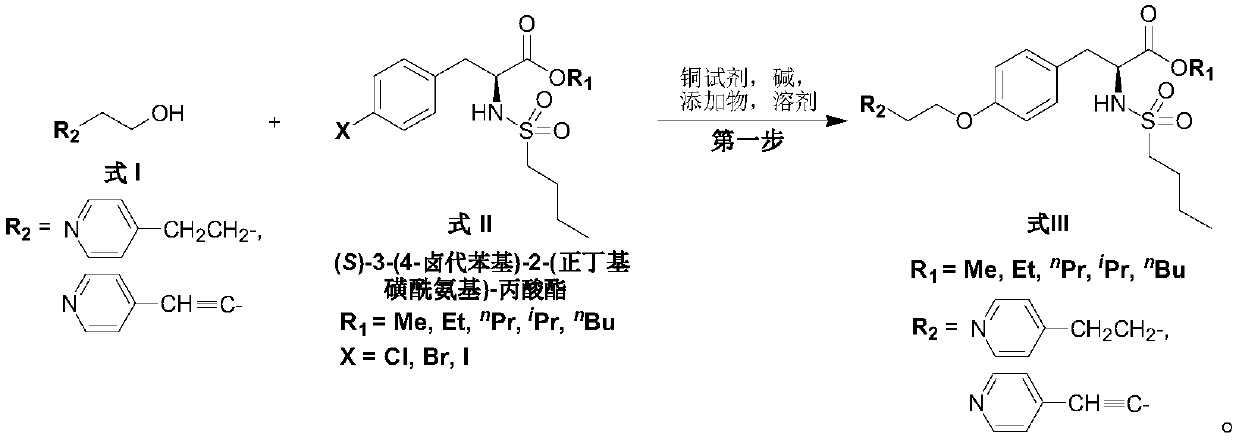
Synthesis of tirofiban hydrochloride
ActiveCN111233748AEasy to operateEasy to realize industrializationOrganic chemistry methodsPropanoic acidPhenyl group
The invention relates to a method for preparing tirofiban hydrochloride. The method comprises the following steps: carrying out a condensation reaction between 4-pyridine butanol or 4-(pyridine-4-yl)-butyl-3- alkynyl-1-ol and (S)-3-(4-halogenated phenyl)-2-(n-butylsulfonylamino)-propionate in the presence of a copper reagent / alkali / additive / solvent to generate (S)-2-(n-butylsulfonylamino)-3-(4-(4-(pyridine-4-yl)butoxy)phenyl)-propionate or (S)-2-(n-butylsulfonylamino)-3-(4-((4-(pyridine-4-yl)-butyl-3-alkynyl-1-yl)oxo) phenyl) propionate.
Owner:WISDOM PHARM CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com