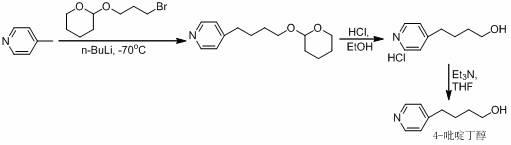
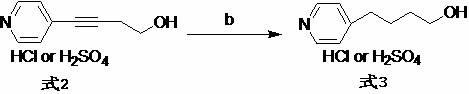
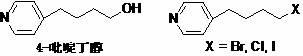A New Process for the Preparation of 4-Pyridine Butanol
A technology of pyridine butanol and new process, applied in the direction of organic chemistry, etc., can solve problems such as instability, difficult storage, rising cost, etc., and achieve the effects of good stability, easy purification, and convenient storage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0026] 1. Preparation of 4-(4-pyridyl)-3-butyn-1-alcohol hydrochloride
[0027] Add 4-bromopyridine hydrochloride (45.0 g), triethylamine (450 mL), and deionized water (90 mL) into the reaction flask in sequence, and stir to dissolve. Lithium chloride (0.5g), cuprous iodide (0.5g) and 3-butyn-1-ol (19.5g) were added, and the reaction mixture was stirred for 0.5h, and tetrakis(triphenylphosphine)palladium (0.1g ), back flow reaction 3h. Cool down to room temperature, separate the organic layer, extract the aqueous layer with an organic solvent, combine the organic layers, and distill off the solvent under reduced pressure to obtain a light brown oily liquid (34.5g). The resulting oil was directly dissolved in ether (200mL), and 6mol.L was added dropwise under ice-cooling -1 Hydrogen chloride ethanol solution (43mL), stirred in ice bath for 2h. Filter and dry to obtain a white powdery solid (36.4g). TLC detected no impurities (R f =0.6, developer: ethyl acetate, sample pre-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com