Tirofiban hydrochloride freeze-dried powder injecta and preparing method
A technology of tirofiban and needle injection, which is applied in the field of pharmaceutical preparations and its preparation, can solve the problems of solution-insoluble particles, inconvenient transportation, large volume, and heavy weight, and achieve easy storage and transportation, reduced transportation costs, and obvious economic benefits Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] prescription
[0024] Tirofiban hydrochloride monohydrate 5.62g
[0025] 200g lactose
[0026] Made into 1000
[0027] Take prescription amount of lactose, add 1600ml water for injection, stir to dissolve, add 0.1% (g / ml) activated carbon for needles, stir at 50℃ for 30 minutes, filter and decarbonize, add prescription amount of tirofiban hydrochloride, stir Dissolve, add water for injection to 2000ml, adjust the pH of the solution to 2.5 with hydrochloric acid or sodium hydroxide, sterile precision filtration with 0.22μm microporous membrane, determine the content of semi-finished products, quantitatively fill in vials, stopper, freeze Dry, sterile full stopper, pressed aluminum plastic cover, packaged.
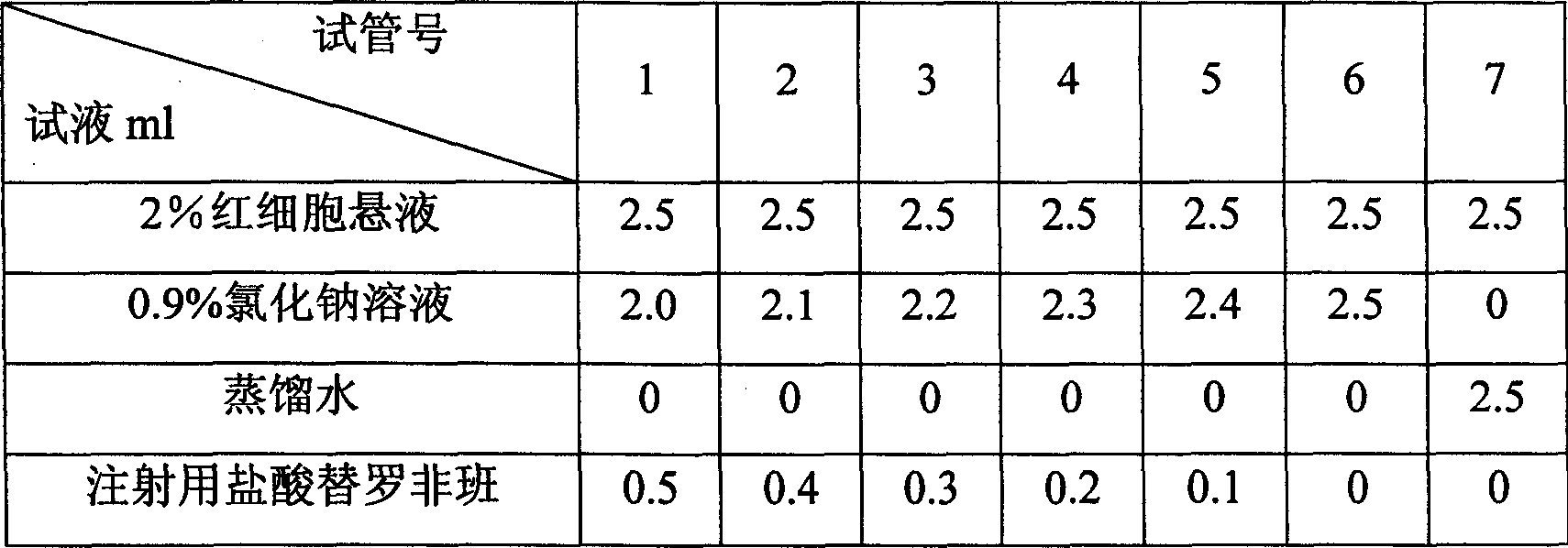
[0028] Inspection: 1. Visible foreign body: According to the method of Appendix IX H of the Chinese Pharmacopoeia 2005 Edition, it meets the requirements.
[0029] 2. Insoluble particles: According to the method of Appendix IX C of the Chinese Pha...
Embodiment 2
[0031] prescription
[0032] Tirofiban hydrochloride monohydrate 5.62g
[0033] Lactose 75g
[0034] Made into 1000
[0035] Take prescription amount of lactose, add 1200ml water for injection, stir to dissolve, add 0.01% (g / ml) activated carbon for needles, stir at 25℃ for 60 minutes, filter and decarbonize, add prescription amount of tirofiban hydrochloride, stir Dissolve, add water for injection to 1500ml, adjust the pH of the solution with hydrochloric acid to 2, 0.22μm microporous filter membrane aseptically and finely filter, determine the content of semi-finished products, quantitatively fill in vials, stopper, freeze-dry, sterile Fully press stopper, press aluminum and plastic cover, ready to pack.
[0036] Inspection: 1. Visible foreign body: According to the method of Appendix IX H of the Chinese Pharmacopoeia 2005 Edition, it meets the requirements.
[0037] 2. Insoluble particles: According to the method of Appendix IX C of the Chinese Pharmaco...
Embodiment 3
[0039] prescription
[0040] Tirofiban hydrochloride monohydrate 5.62g
[0041] 800g lactose
[0042] Made into 1000
[0043] Take prescription amount of lactose, add 3200ml water for injection, stir to dissolve, add 0.5% (g / ml) activated carbon for needles, stir at 100°C for 10 minutes, filter and decarbonize, add prescription amount of tirofiban hydrochloride, stir Dissolve, add injection water to 4000ml, adjust the pH of the solution to 3 with sodium hydroxide, aseptically filter with 0.22μm microporous membrane, determine the content of semi-finished products, quantitatively fill in vials, stopper, freeze-dry, Sterile full stopper, pressed aluminum plastic cover, packaged.
[0044] Inspection: 1. Visible foreign body: According to the method of Appendix IX H of the Chinese Pharmacopoeia 2005 Edition, it meets the requirements.
[0045] 2. Insoluble particles: According to the method of Appendix IX C of the Chinese Pharmacopoeia 2005 edition, it meets t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com