Synthesis method of tirofiban hydrochloride intermediate III
A technology of tirofiban and a synthesis method, applied in the field of synthesis of tirofiban hydrochloride intermediate III, can solve the problems of long production cycle, unsuitable for industrialized production, complicated operation, etc., and achieves reduction of reaction time and enhancement of π-surface. Selective, responsive effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
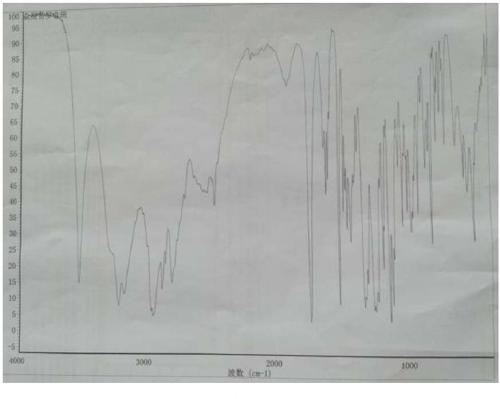
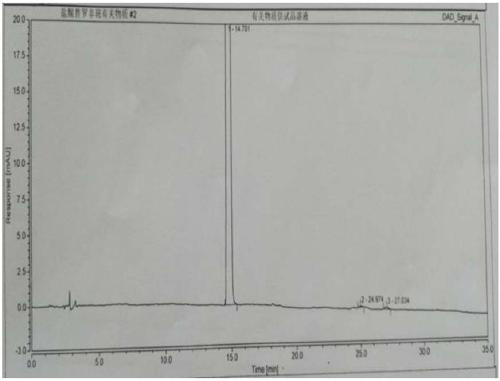
Image
Examples
Embodiment 1
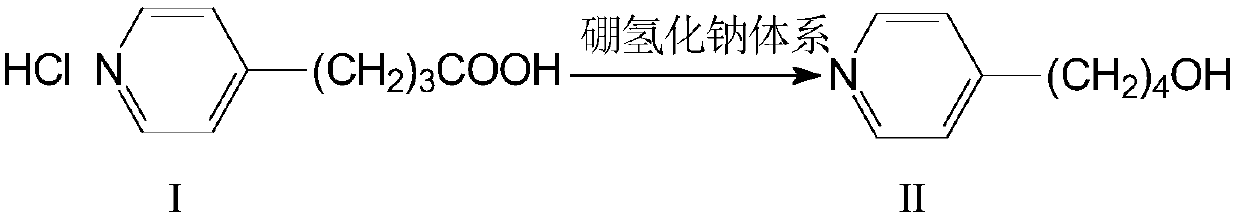
[0060] Example 1 Preparation of 4-(4-pyridyl)-1-butanoic acid (intermediate I)
[0061] Under the protection of nitrogen, add 23g of sodium metal cut into small pieces into 600ml of absolute ethanol in batches, and quickly add 470ml of diethyl malonate after completely dissolving, stir for 10 minutes, add 210ml of 4-vinylpyridine dropwise, and the reaction is complete. Distill ethanol off under reduced pressure, add 400ml of water, acidify, extract with ethyl acetate, add solid NaOH to the aqueous phase to alkalinize and hydrolyze overnight, extract with dichloromethane, acidify and decarboxylate the aqueous phase, evaporate water under normal pressure until solidified, and then use 95% ethanol Reflux extraction, decolorization, evaporation to dryness, acetone recrystallization, and drying at 70°C gave 230 g of intermediate I with a yield of 80%. Part of the product can be recovered from the mother liquor.
Embodiment 2
[0062] Example 2 Preparation of 4-(4-pyridyl)-1-butanol (intermediate II)
[0063] Add 1.5L of tetrahydrofuran and 150g of sodium borohydride to a dry container, add 300g of intermediate I in batches, stir and react for 0.5 hours after the addition, add 100g of concentrated sulfuric acid dropwise to keep the temperature below 10°C, be careful not to add too quickly to prevent Wash the material, slowly raise the temperature to reflux for 10 hours, then drop the temperature and add hydrochloric acid, separate the upper layer solution, add water and adjust the pH value to 9 with caustic soda, then extract with ethyl acetate, dry the organic layer with anhydrous magnesium sulfate, filter and dry agent, and evaporated to dryness under reduced pressure to obtain 209g of yellow liquid intermediate II with a yield of 90.1%. Developing solvent: ethyl acetate / n-hexane=1 / 1.
Embodiment 3
[0064] Example 3 Preparation of 4-(4-pyridyl)-1-butanol (intermediate II)
[0065] Add 1.5 L of tetrahydrofuran and 150 g of sodium borohydride to a dry container, add 300 g of intermediate I and 110 g of ZnCl in batches 2 , slowly warming up to reflux for 8 hours, then dropping the temperature and adding hydrochloric acid dropwise, separating the upper layer solution, adding water and using sodium carbonate to adjust the pH value to 10, then extracting with ethyl acetate, drying the organic layer with anhydrous magnesium sulfate, and filtering off the desiccant , and evaporated to dryness under reduced pressure to obtain 212 g of yellow liquid intermediate II with a yield of 91%. Developing solvent: ethyl acetate / n-hexane=1 / 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com