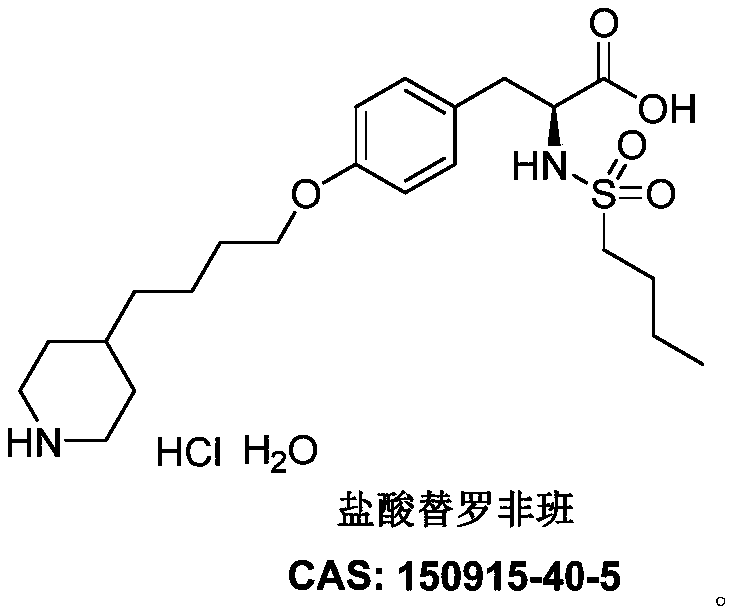
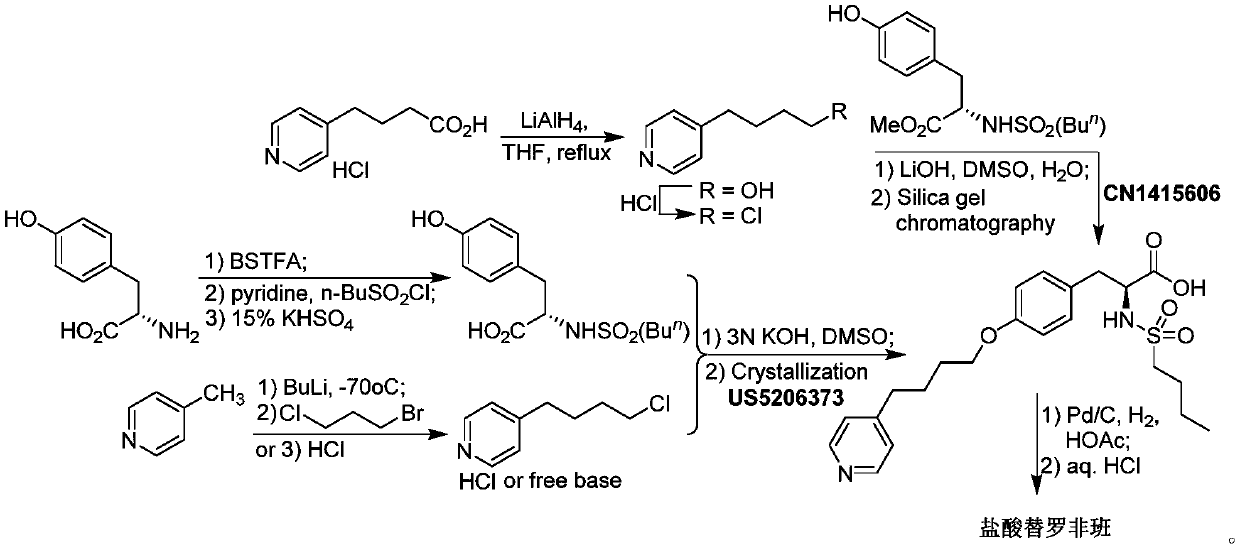
Synthesis of tirofiban hydrochloride
A technology of propionate and condensation reaction, applied in the direction of organic chemistry, organic chemistry method, etc., can solve the problems of complicated reaction operation, triphenylphosphine oxide by-products, etc., and achieve the effect of simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
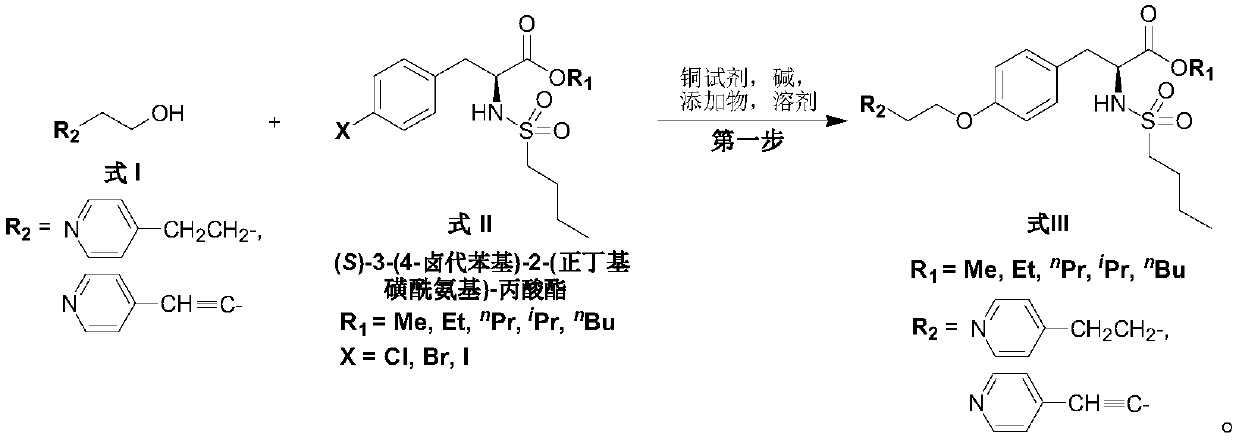
[0032] 1. Preparation of (S)-2-(n-butylsulfonylamino)-3-(4-(4-(pyridin-4-yl) butyl oxygen) phenyl) propionic acid ethyl ester (formula III, R 1 =Et,R 2 = ).
[0033] (S)-3-(4-chlorophenyl)-2-(n-butylsulfonylamino)-propionic acid ethyl ester (139.0g, 0.40mol), 4-pyridinebutanol (181.5g, 1.20mol) , CuI (3.80g, 19.95mmol), K 3 PO 4 (127.4g, 0.60mol) and N 1 ,N 2 - bis(naphthalene-1-ylmethyl) oxalamide (14.8g, 39.96mmol) was added to the reaction flask, followed by nitrogen replacement three times, then anhydrous DMSO (500mL) was added to the reaction flask, and the reaction system was again nitrogen Replace three times. Subsequently, the reaction system was heated to 100° C. for 24 h under stirring. After the reaction was over, the system was naturally cooled to room temperature. The reaction system was diluted with ethyl acetate (1.5 L), then filtered through celite. The filtrate was desolvated under reduced pressure to remove the organic solvent. The residue was add...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com