Tirofiban hydrochloride injection and preparation method thereof
A technology for tirofiban and injection, which is applied in the field of tirofiban hydrochloride injection and its preparation, can solve problems such as hidden safety hazards, increased substance content, unstable properties, etc., and achieves good effect, high active substance content, Simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
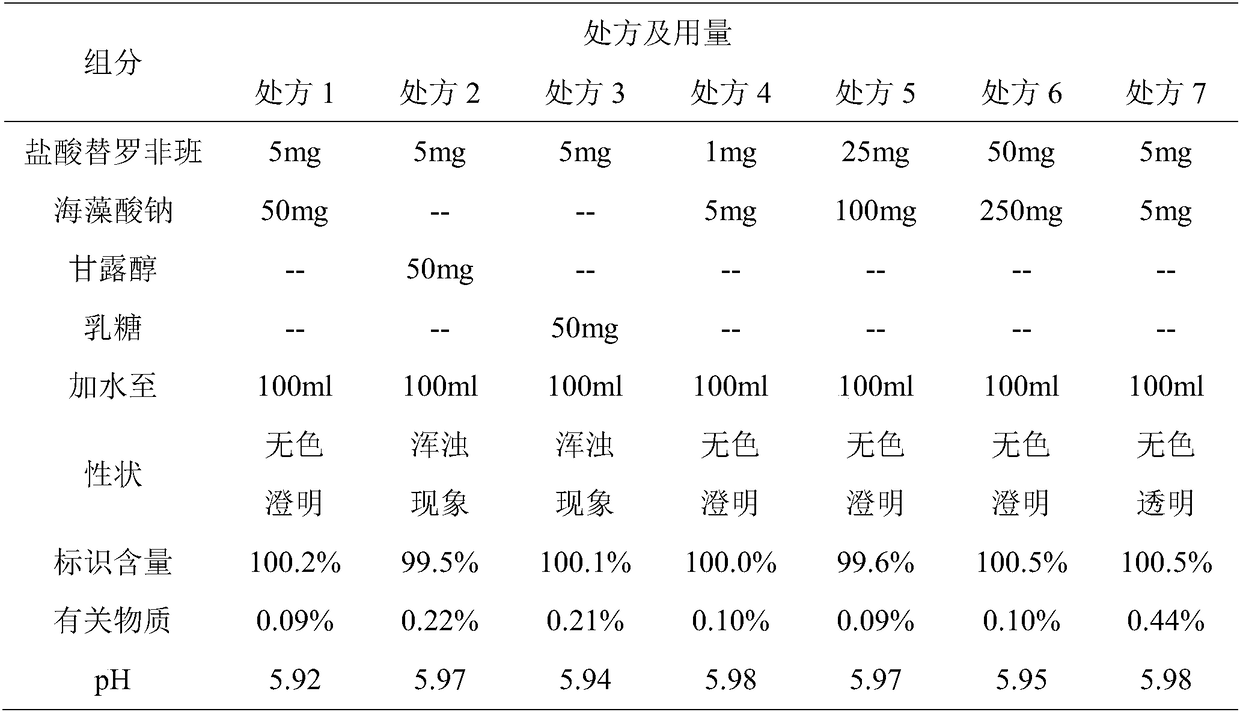
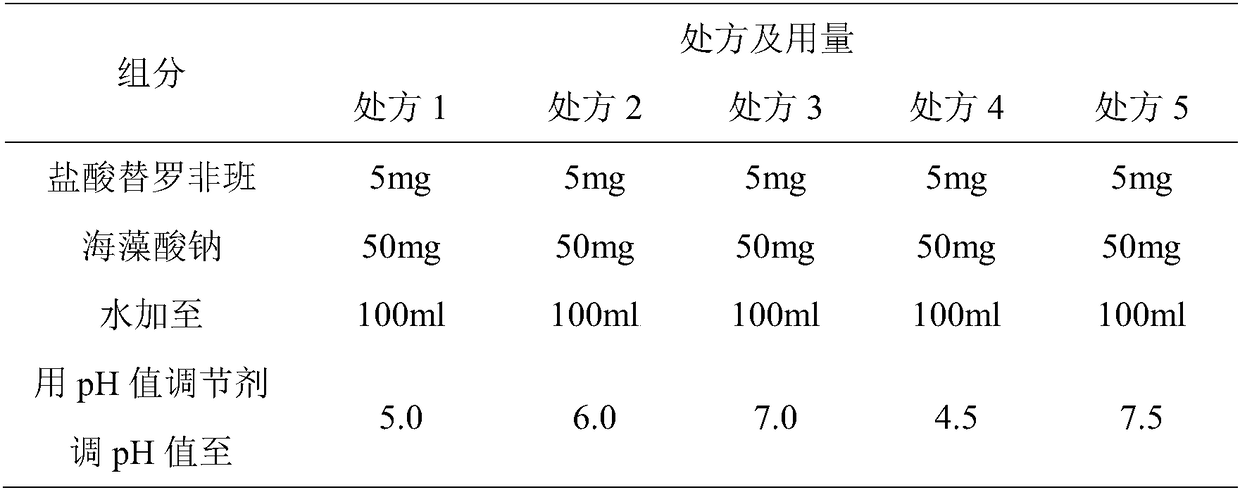
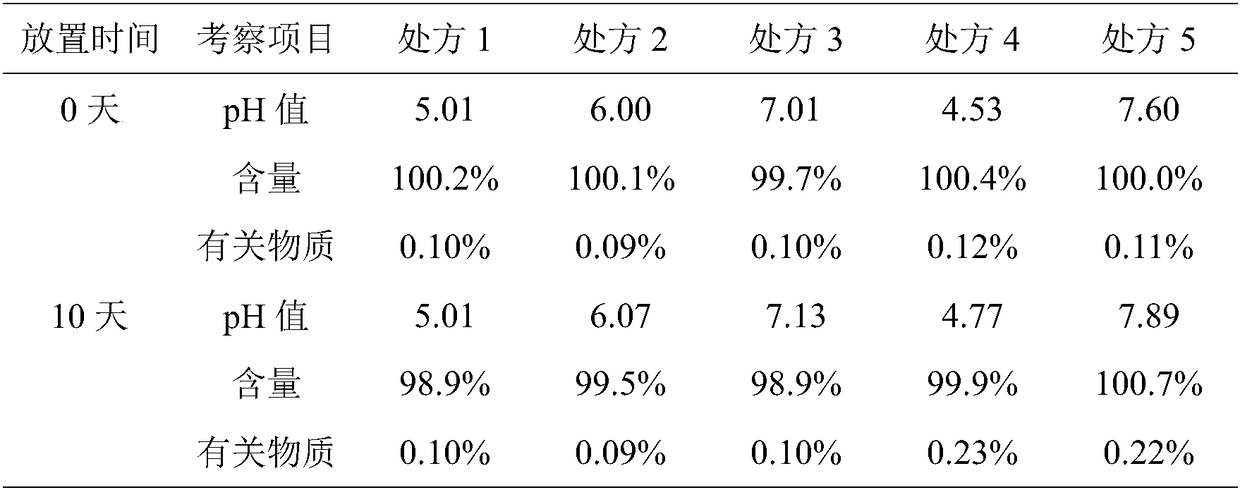
[0041] Embodiment 1: 10 bottles of tirofiban hydrochloride prescription and its preparation method are as follows:
[0042] prescription:
[0043] Tirofiban Hydrochloride 50mg
[0044] Sodium Alginate 500mg
[0045] Appropriate amount of PH regulator
[0046] Preparation Process:
[0047] 1) Dissolving citric acid and citric acid in 30% water to prepare a citric acid-sodium citrate buffer solution for later use;
[0048] 2) Dissolve tirofiban hydrochloride and sodium alginate in 50% water, mix with the buffer solution in step 1), set the volume to 1000ml in full, and set aside;
[0049] 3) Use dipotassium hydrogen phosphate to adjust the pH value of the mixture obtained in step 2) to 6.0, filter it through a 0.22 μm microporous membrane, and pack it into glass infusion bottles, stopper, cap, sterilize, light check, and pack. .
Embodiment 2
[0050] Embodiment 2: 10 bottles of tirofiban hydrochloride prescription and its preparation method are as follows:
[0051] prescription:
[0052] Tirofiban Hydrochloride 250mg
[0053] Sodium Alginate 1500mg
[0054] Appropriate amount of PH regulator
[0055] Preparation Process:
[0056] 1) Dissolving citric acid and citric acid in 30% water to prepare a citric acid-sodium citrate buffer solution for later use;
[0057] 2) Dissolve tirofiban hydrochloride and sodium alginate in 50% water, mix with the buffer solution in step 1), set the volume to 1000ml in full, and set aside;
[0058]3) Use dipotassium hydrogen phosphate to adjust the pH value of the mixture obtained in step 2) to 6.0, filter it through a 0.22 μm microporous membrane, and pack it into glass infusion bottles, stopper, cap, sterilize, light check, and pack. .
Embodiment 3
[0059] Embodiment 3: 10 bottles of tirofiban hydrochloride prescription and its preparation method are as follows:
[0060] prescription:
[0061] Tirofiban Hydrochloride 10mg
[0062] Sodium Alginate 50mg
[0063] Appropriate amount of PH regulator
[0064] Preparation Process:
[0065] 1) Dissolving citric acid and citric acid in 30% water to prepare a citric acid-sodium citrate buffer solution for later use;
[0066] 2) Dissolve tirofiban hydrochloride and sodium alginate in 50% water, mix with the buffer solution in step 1), set the volume to 1000ml in full, and set aside;
[0067] 3) Use dipotassium hydrogen phosphate to adjust the pH value of the mixture obtained in step 2) to 6.0, filter it through a 0.22 μm microporous membrane, and pack it into glass infusion bottles, stopper, cap, sterilize, light check, and pack. .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com