Process for preparation of tirofiban hydrochloride
A technology for tirofiban and hydrochloric acid, which is applied in the field of producing tirofiban hydrochloride, can solve the problems of high risk, harsh reaction conditions, troublesome operation, etc., and achieve the effects of less discharge of three wastes, no risk of operation, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0021] The preparation method of the present invention is further described in detail below by way of examples.
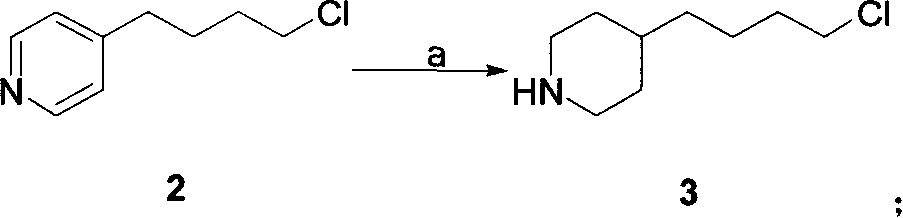
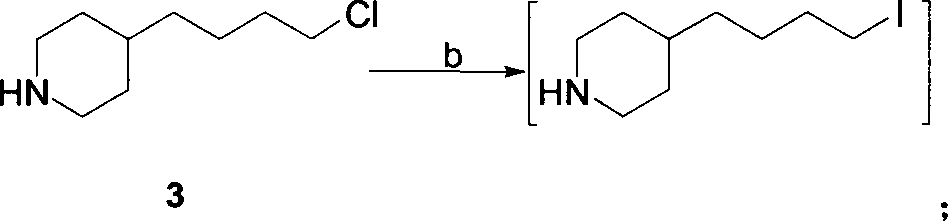
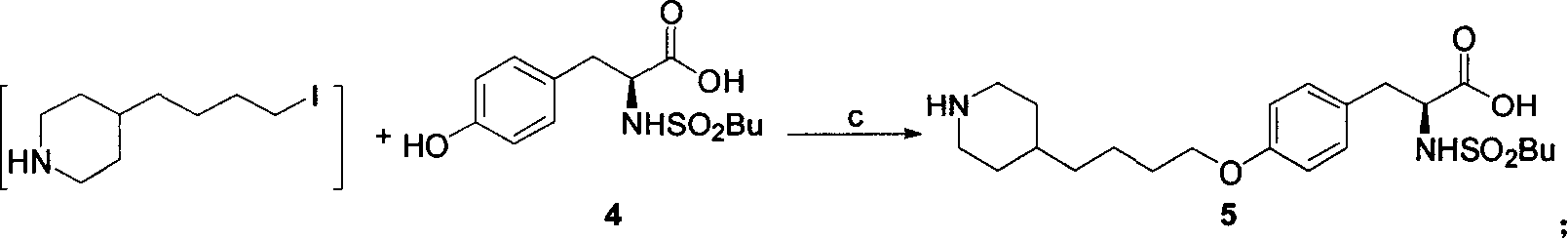
[0022] 1. Preparation of 4-(4-piperidinyl)butyl chloride 3:
[0023] Method A: 4-(4-pyridyl) butyl chloride (507 grams, 3 moles), 10% Pd / C (25.4 grams, 5% by weight) were dissolved in 6 liters of ethanol, at a hydrogen pressure of 0.35 MPa and 65 ℃, hydrogenation to complete (about 8 hours). The catalyst was removed by filtration, and the filtrate was concentrated under reduced pressure to obtain a colorless viscous liquid, which was 4-(4-piperidinyl)butyl chloride, about 525 g.
[0024] Method B: 4-(4-pyridyl)butyl chloride (507 g, 3 moles) was dissolved in 6 liters of absolute ethanol, heated to reflux, and sodium metal (575 g, 25 moles) was added. After the reaction, most of the ethanol was evaporated, cooled to room temperature, 500 ml of saturated ammonium chloride aqueous solution was added, extracted with ethyl acetate (500 ml × 3), the ethyl acetate layer...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com