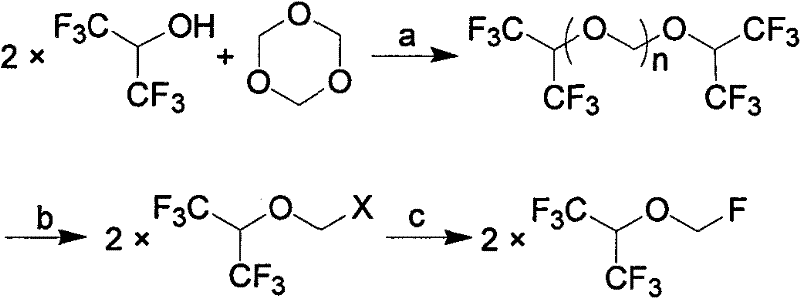
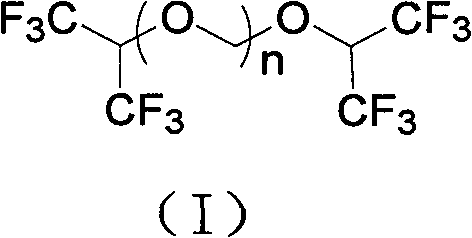
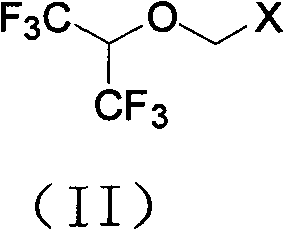
Process for synthesizing Sevoflurane
一种合成方法、七氟烷的技术,应用在化学仪器和方法、有机化合物的制备、有机部分交换制备醚等方向,能够解决六氟异丙醇转化率低、增加制造成本、七氟烷杂质多等问题,达到操作简便无危险、原材料消耗低、三废排放少的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Add 336g of hexafluoroisopropanol, 90g of paraformaldehyde, and 15ml of concentrated sulfuric acid into the reaction flask, stir and heat to 40°C~80°C for 15 hours, let stand to separate layers, remove concentrated sulfuric acid, stir and cool down to 0°C for crystallization 3 hour, filter, and the filtrate is 159.6g, and this filtrate is detected by gas chromatography (GC), and is confirmed to be the mixture of hexafluoroisopropanol and paraformaldehyde, which can be directly used as the reaction stock solution after simple filtration; The dried solid weighed 247.2g. GC detected that in the retentate, the content of bis-hexafluoroisopropanol triformal (n=3) was 52%, and the content of bis-hexafluoroisopropanol triformal (n=2 ) content is 42%, and bis-hexafluoroisopropanol-formal (n=1) content is <0.5%.
Embodiment 2
[0033] Take 123.6g of the solid obtained in Example 1 and add it to the reaction flask, heat to 40-45°C to dissolve, cool down to 20-25°C, add anhydrous aluminum trichloride (82.5g) in batches, and keep warm at 25-30°C for 24 Hours, cool down to 0°C, add 10% dilute hydrochloric acid (500ml) dropwise, let stand to separate layers, wash the organic layer successively with 5% NaOH aqueous solution (100ml×2), water (100ml×2), and dry over anhydrous sodium sulfate , to obtain 108.5 g of chloromethyl 2,2,2-trifluoro-1-(trifluoromethyl)ethyl ether, purity: 99.3% (GC method).
Embodiment 3
[0035]Get 123.6 g of the solid obtained in Example 1 and put it into the reaction flask, and add 100 ml of dichloromethane, add anhydrous aluminum trichloride (82.5 g) in batches at 0 ° C, keep warm at 25 ~ 30 ° C for 24 hours, and cool down to 0°C, add 10% dilute hydrochloric acid (500ml) dropwise, let stand to separate layers, wash the organic layer with 5% NaOH aqueous solution (100ml×2), water (100ml×2) successively, dry over anhydrous sodium sulfate, rectify The dichloromethane was removed to obtain 109.2 g of chloromethyl 2,2,2-trifluoro-1-(trifluoromethyl)ethyl ether with a purity of 97.3% (GC method).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com