A kind of preparation method of tirofiban hydrochloride
A technology of tirofiban and hydrochloric acid, which is applied in the field of preparation of tirofiban hydrochloride, can solve the problems of high potential safety hazards, troublesome solvent recovery and treatment, and increased cost, and achieve improved safety, low-carbon and environmentally friendly reaction conditions, The effect of simple salt-forming conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
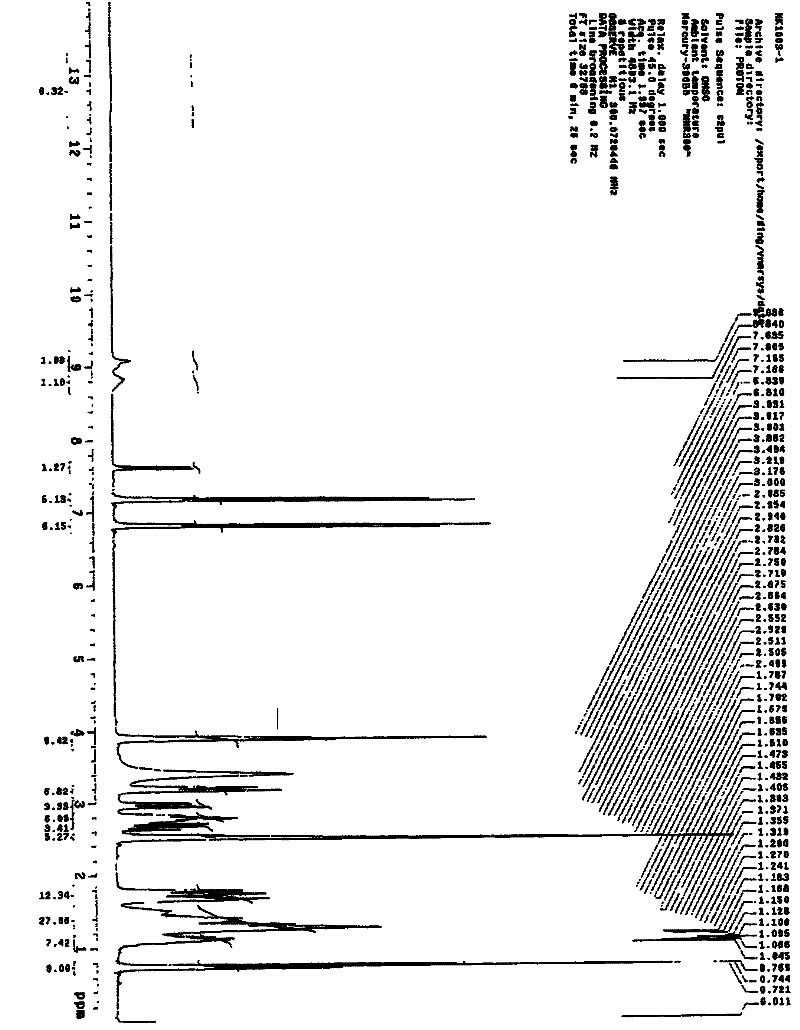
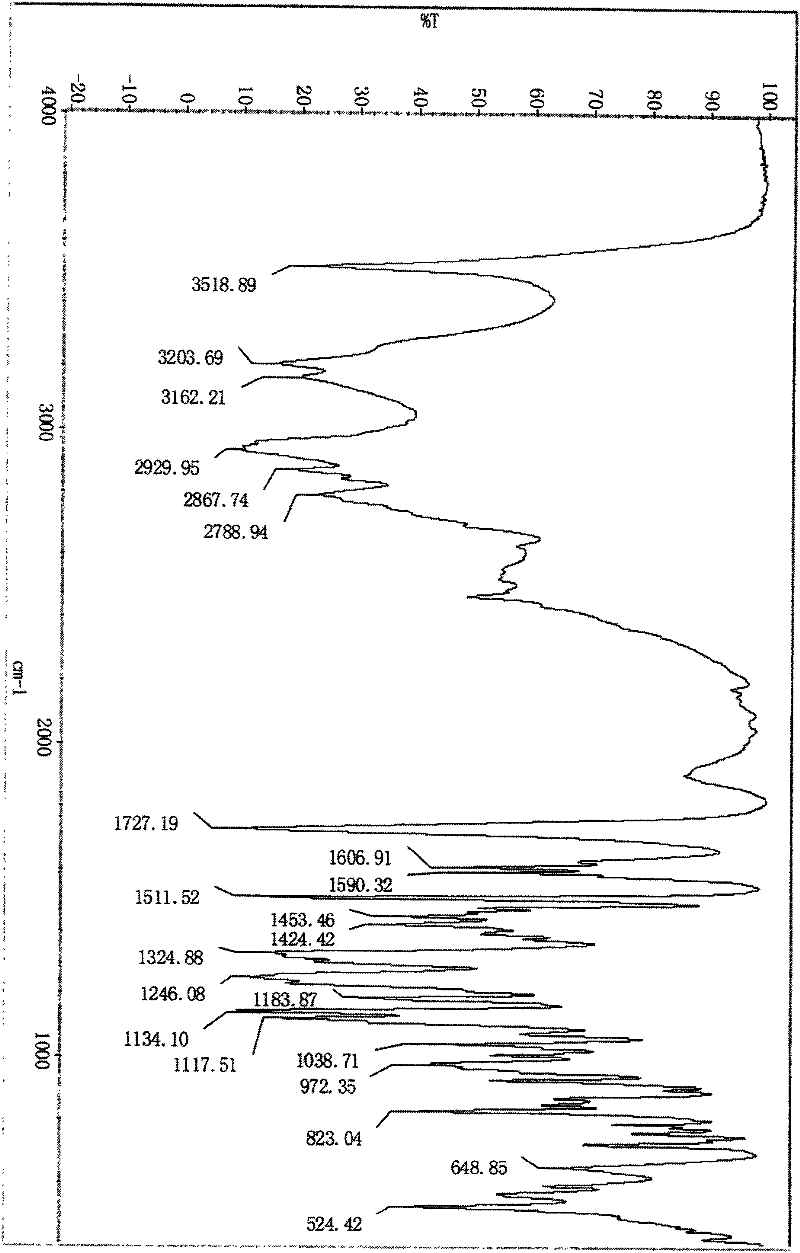
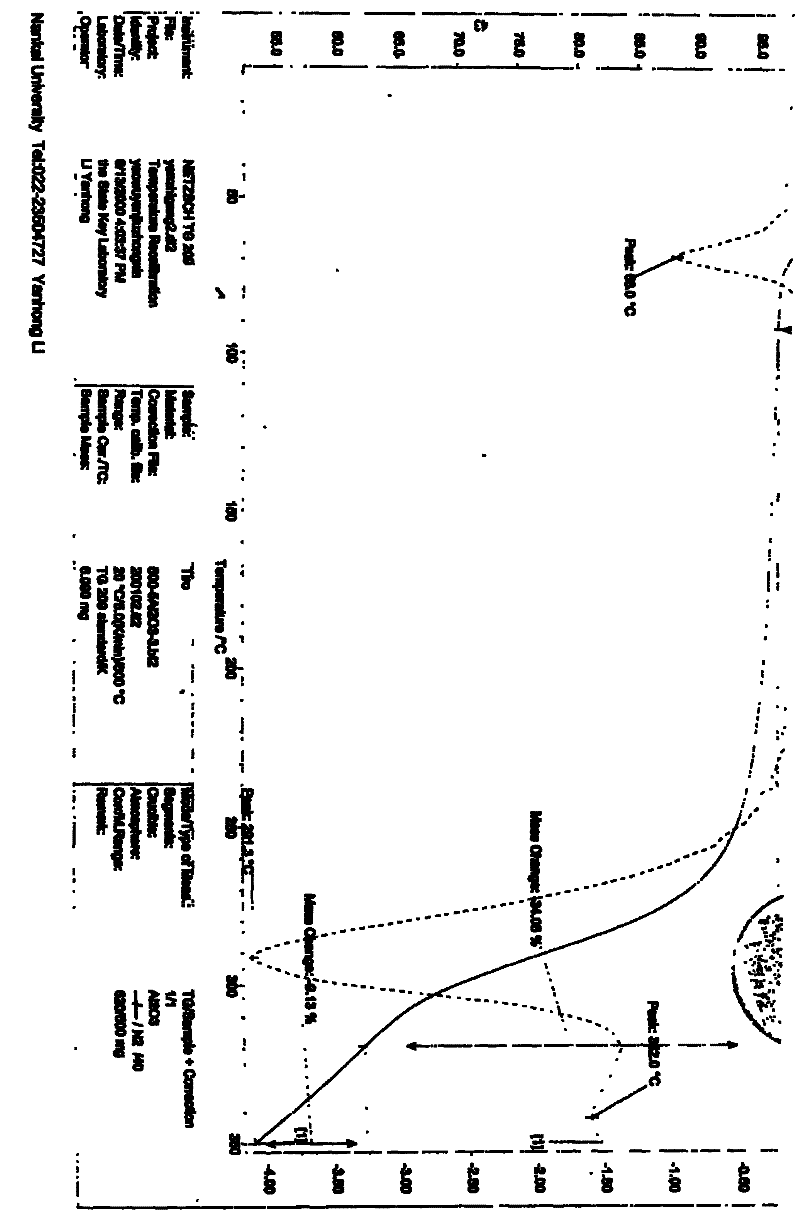
Image
Examples
Embodiment 1
[0018] Weigh 220.0 g of tirofiban, add 4.4L of 1.2mol / L hydrochloric acid to the reaction vessel, heat to 80°C under stirring, to completely dissolve the tirofiban, and keep stirring for 30 minutes after dissolving. Add 2.2 g of activated carbon and stir at 80°C for 30 minutes to decolorize. Filtrate under reduced pressure while hot, cool to 18°C with stirring, and maintain crystallization for 24 hours. The crystals were separated and washed with purified water to obtain a white solid, which was dried at 40-50°C to constant weight. The pulverized package can be directly used as the raw material for injection preparations.
Embodiment 2
[0020] Weigh 220.0 grams of tirofiban into the reaction bottle, add 6.6L of 0.5mol / L hydrochloric acid, and heat to 100±2°C under stirring to completely dissolve the tirofiban. After dissolving, keep stirring for 30 minute. Add 2.2 g of activated carbon for needles and stir at 100±2°C for 30 minutes to decolorize. Filtrate under reduced pressure while hot to another reaction flask. It was cooled to 30°C under stirring, and a large amount of solids precipitated out. Stir at 30°C±2°C for 24 hours. Filter under reduced pressure, wash with 200 mL of purified water, and repeat the washing 3 times to obtain a white solid. Spread the solid out and dry at 40-50°C until constant weight. The pulverized package can be directly used as the raw material for injection preparations.
Embodiment 3
[0022] Weigh 220.0 grams of tirofiban, and add 2.20L of 1.5mol / L hydrochloric acid to the reaction bottle, heat to 75±2°C under stirring to completely dissolve the tirofiban, and keep stirring for 30 minutes after dissolving. minute. Add 2.2 g of activated carbon for needles, and stir at 75±2°C for 30 minutes to decolorize. Filtrate to another reaction bottle under reduced pressure while hot, and cool to 0°C while stirring, a large amount of solids precipitate out. Stir at 0±2°C for 24 hours. Filter under reduced pressure, wash with 200 mL of purified water, and repeat the washing 3 times to obtain a white solid. Spread the solid out and dry at 40-50°C until constant weight. The pulverized package can be directly used as the raw material for injection preparations.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com