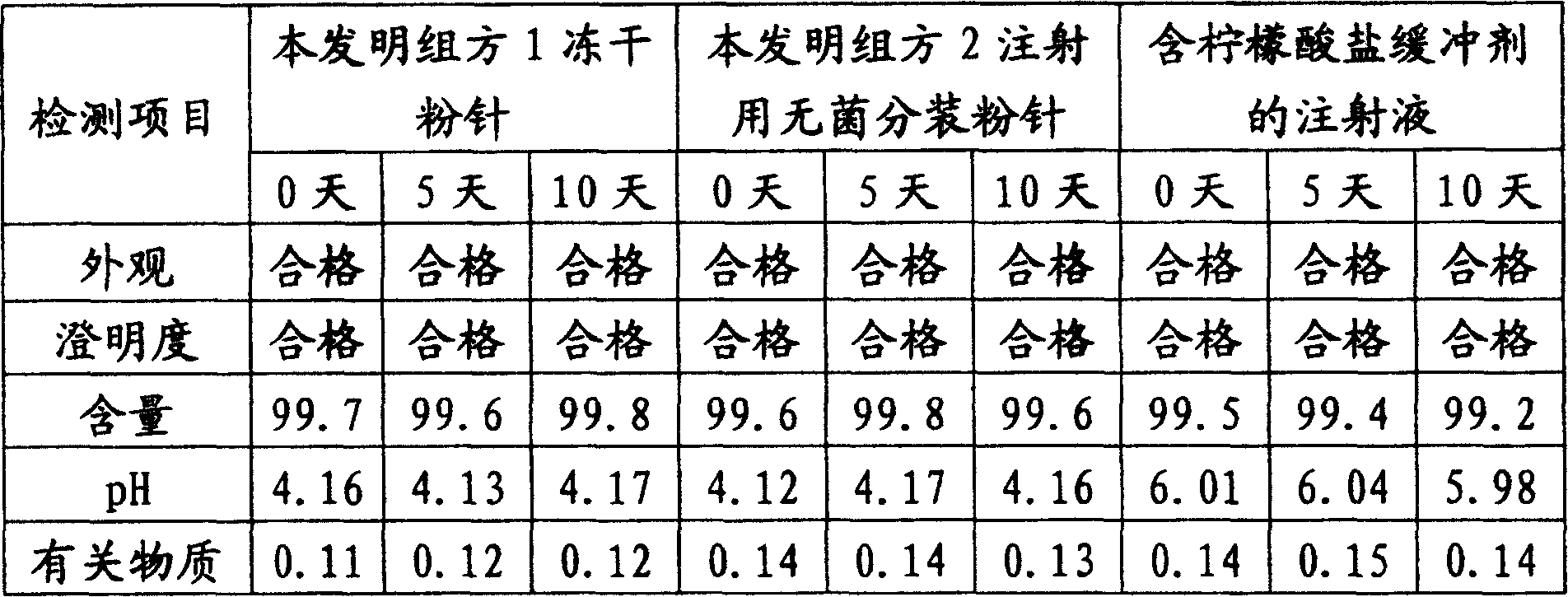
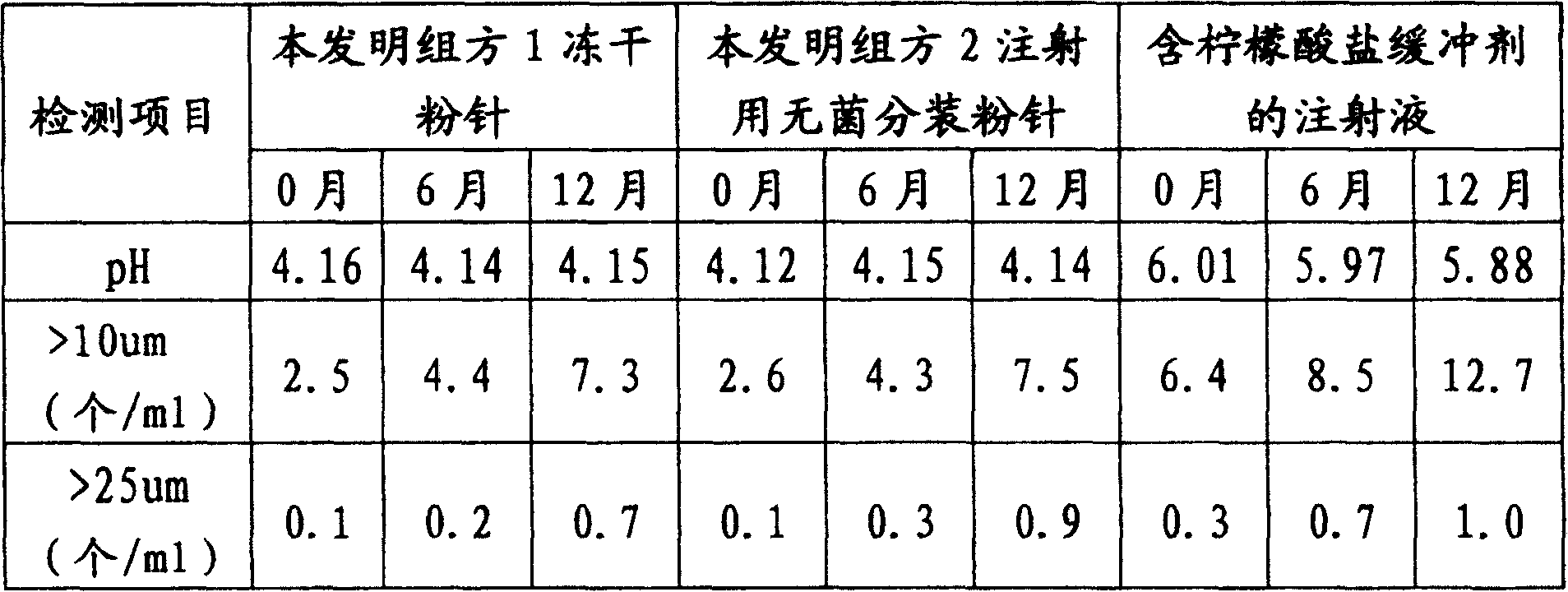
Injection use-powder ampoule for inhibiting thrombocyte agglutination and its preparation method
A technology for platelet aggregation and injection, which is applied in blood diseases, powder delivery, drug combination, etc., can solve the problems of insoluble particles and other problems, and achieve good stability, definite curative effect, and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0011] Embodiment 1 Prescription freeze-dried powder for injection of the present invention
[0012] Specification: 5mg / bottle (calculated as anhydrous tirofiban), the same below
[0013] Tirofiban Hydrochloride 5.618g
[0014] Mannitol 100g
[0015] Water for injection to 2000ml
[0016] A total of 1000 powder needles were made
[0017] Preparation Process:
[0018] Preparation of liquid medicine:
[0019] Take tirofiban hydrochloride and selected pharmaceutically acceptable carrier by prescription quantity, add in 1950ml water for injection, and add water to nearly full amount; After adding water for injection to the prescribed amount, measure the intermediate content and should be 93.0%~107.0%, After passing the test, filter it with a 0.45um microporous membrane, check the clarity and pass it, then pack it into controlled antibiotic bottles, and hand it over to the freeze-drying group.
[0020] Freeze-dried:
[0021] First cool down for 1 hour, the temperature drops ...
Embodiment 2
[0022] Embodiment 2 Prescription injection of the present invention aseptic subpackage powder injection
[0023] Tirofiban Hydrochloride 5.618g
[0024] Mannitol 100g
[0025] A total of 1000 powder needles were made
[0026] Preparation Process:
[0027] Weigh tirofiban hydrochloride and selected pharmaceutically acceptable carrier according to the prescription quantity, add in 300ml ethanol solution and dissolve, ethanol: water is 95:5, under sterile conditions, filter with 0.45um microporous membrane, check clarity After passing the test, dry it; the content of the inspection intermediate should be 93.0% to 107.0%, and after passing the ethanol residue, pack it into a controlled antibiotic bottle and press the composite aluminum cover.
Embodiment 3
[0028] Embodiment 3 contrasts with the injection containing citrate buffer
[0029] Tirofiban Hydrochloride 5.618g
[0031] Sodium citrate 54g
[0032] Citric acid 3.2g
[0033] A total of 1000 bottles of injection were made
[0034] Preparation Process:
[0035] Liquid preparation:
[0036] Weigh sodium chloride, citric acid and sodium citrate according to the prescription amount, add in 5000ml of newly prepared water for injection, stir until completely dissolved; weigh the active carbon of 0.3% solution amount, stir well, heat and boil for 15 minutes, after cooling, filter Remove activated carbon; accurately weigh tirofiban hydrochloride according to the prescription amount, completely dissolve it with water for injection, add it to the above sodium chloride solution, and add water for injection to nearly full amount; measure the pH at 5.5-6.5, add water for injection to the specified amount, Determination of intermediate content should ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com