Separation method for tirofiban hydrochloride isomer, and metering method for D-configuration tirofiban hydrochloride
A technology of tirofiban and a separation method is applied in the field of separation of tirofiban hydrochloride isomers, and can solve the problems of poor separation, poor accuracy, large errors and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
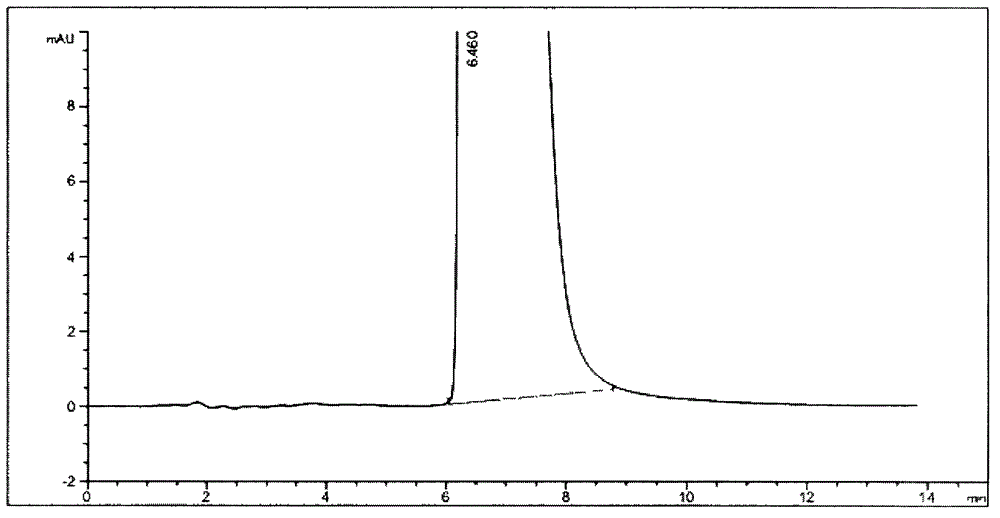
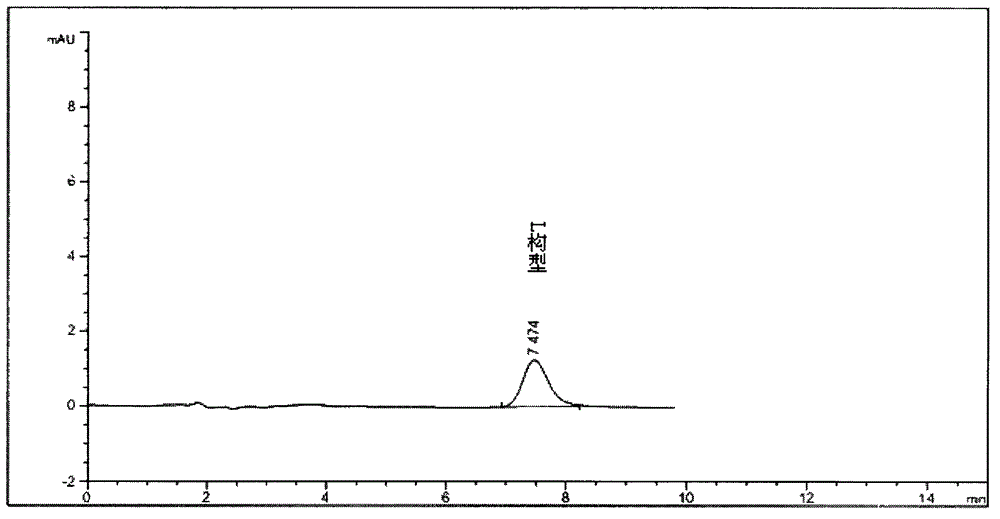
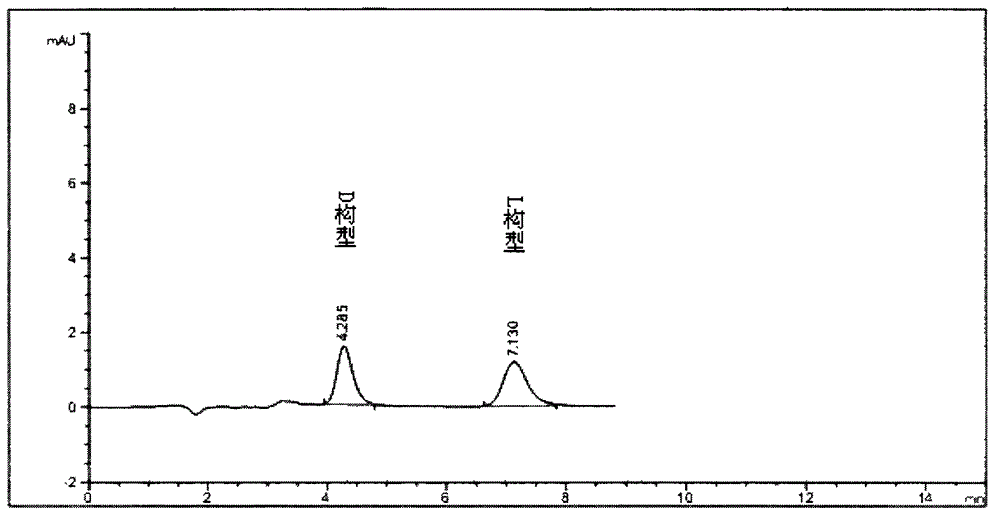
[0080] Use the chiral chromatographic column of ovomucin as the stationary phase; With 0.08% trifluoroacetic acid aqueous solution (regulating the pH value with triethylamine to be 3.0) the volume ratio of water phase and methanol is 99: 1 as mobile phase; 0.8ml per minute; detection wavelength is 230nm.
[0081] Under this condition, detect need testing solution, contrast solution and the detection spectrum that contrast substance solution obtains are similar to Figure 1-1A , Figure 1-1B with 1-1C . After replacing the organic phase with ethanol, isopropanol, acetonitrile or any combination of them, as long as other conditions remain unchanged, the detection results are similar.
Embodiment 2
[0083] Use the chiral chromatographic column of ovomucin as the stationary phase; the volume ratio of 0.10% trifluoroacetic acid aqueous solution (adjusting the pH value to 3.5 with triethylamine) and methanol is 85:15 as the mobile phase; the flow rate is 0.8 per minute ml; detection wavelength is 200nm.
[0084] Under this condition, detect need testing solution, contrast solution and the detection spectrum that contrast substance solution obtains are similar to Figure 1-2A , Figure 1-2B with 1-2C . After replacing the organic phase with ethanol, isopropanol, acetonitrile or any combination of them, as long as other conditions remain unchanged, the detection results are similar.
Embodiment 3
[0086] Use the chiral chromatographic column of ovomucin as the stationary phase; the volume ratio of 0.15% trifluoroacetic acid aqueous solution (adjusting the pH value to 4.0 with triethylamine) and methanol is 80:20 as the mobile phase; the flow rate is 0.8 per minute ml; detection wavelength is 227nm.
[0087] Under this condition, detect need testing solution, contrast solution and the detection spectrum that contrast substance solution obtains are similar to Figure 1-2A , Figure 1-2B with 1-2C . After replacing the organic phase with ethanol, isopropanol, acetonitrile or any combination of them, as long as other conditions remain unchanged, the detection results are similar.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| separation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com