Method for detecting related substances of tirofiban hydrochloride injection
A technology for tirofiban and related substances, which is applied in the detection field of related substances of tirofiban hydrochloride injection, can solve the problems of peak tailing, long retention time of the main peak, failure to meet system requirements, etc., and achieve good accuracy, The effect of strong specificity and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Tirofiban hydrochloride injection and its preparation
[0037] The preparation method is firstly dissolving citric acid and sodium citrate in 10-50% water to prepare a citric acid-sodium citrate buffer solution, then dissolving tirofiban hydrochloride and sodium alginate in water, and the two Mix the solution; adjust the pH, then filter, subpackage, sterilize, light check, and pack.
Embodiment 2
[0038] Embodiment 2 (comparative experiment 1)
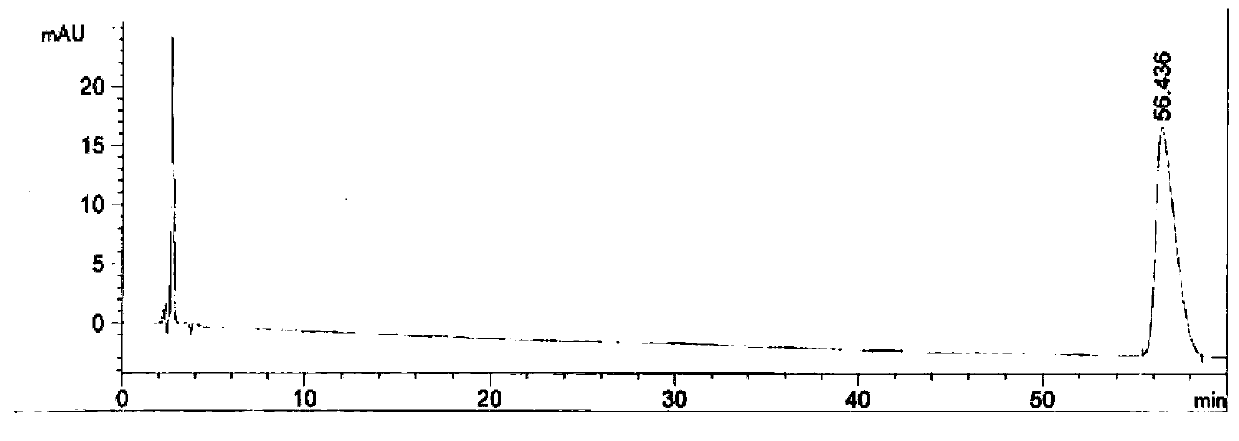
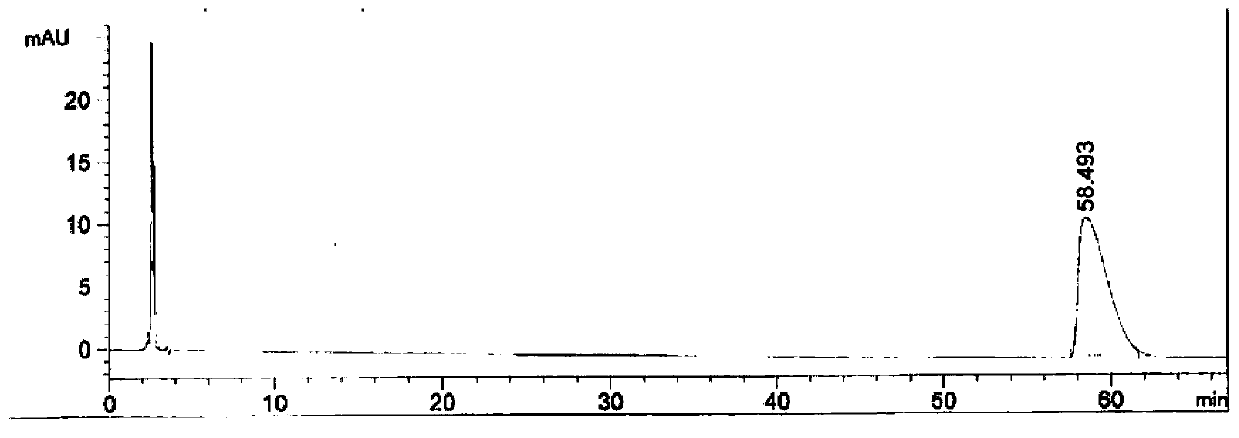
[0039] Adopt the relevant substance detection method in the imported drug registration standard (standard number: JX20080265), select the chromatographic column of different brands to measure the obtained tirofiban hydrochloride injection of embodiment 1, record chromatogram, as figure 1 , figure 2 .
Embodiment 3
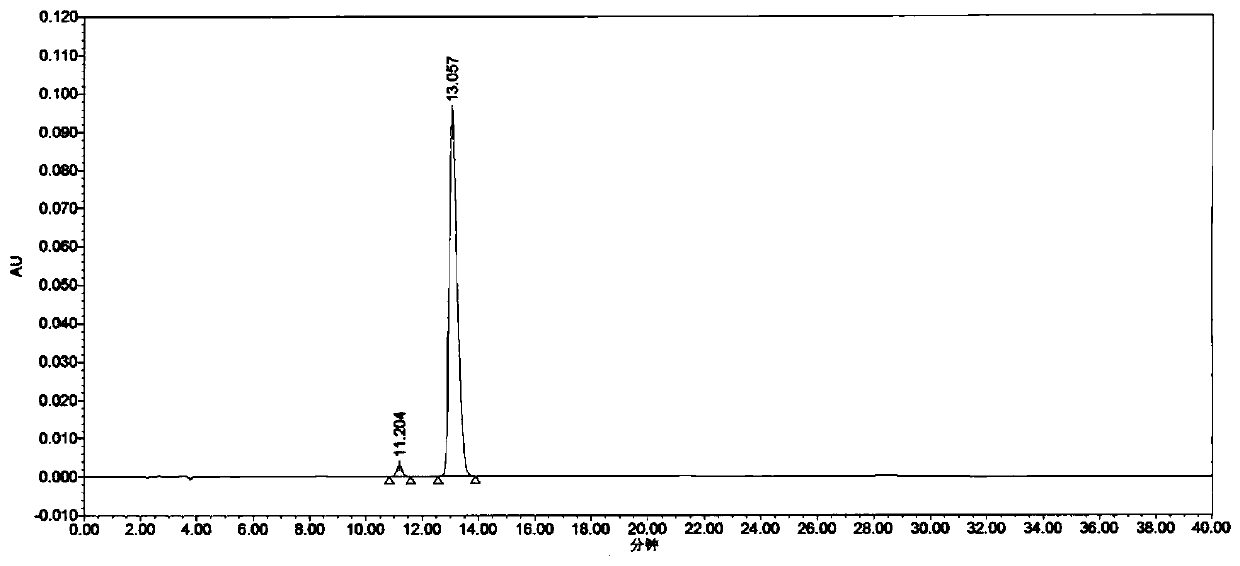
[0041] specificity test
[0042] System suitability test solution: take appropriate amount of tirofiban hydrochloride reference substance and impurity A reference substance, accurately weigh them respectively, add water to dilute and dissolve, and make a mixed solution containing about 25 μg of tirofiban and 0.5 μg of impurity A per 1 ml , as a system suitability test solution.
[0043] Blank solution: Measure 5ml of the blank excipient solution, put it in a 25ml measuring bottle, add water to dilute to the mark, shake well, filter, and take the subsequent filtrate.
[0044] Get the sample in embodiment 1 in right amount, dilute with water and make the solution that contains about tirofiban 25 μ g in every 1ml, as need testing solution.
[0045] Need test solution (oxidative damage): Accurately measure 2ml of sample solution, put it in a 20ml measuring bottle, add 2ml of 10% hydrogen peroxide solution, mix well, heat in a water bath at 95°C for 30 minutes, cool, dilute with w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com