Tirofiban hydrochloride freeze-dried powder injection preparation and preparation method thereof
A technology of tirofiban and freeze-dried powder injection, which is applied in the field of medicine, can solve the problems of lactose without injection specifications, unproven safety, and hidden dangers of medication for patients, and achieve improved solubility, guaranteed safety, and convenient transportation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
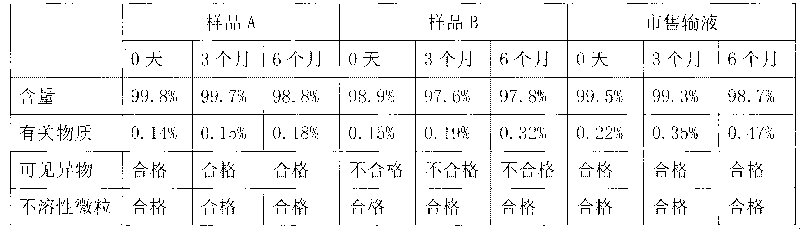
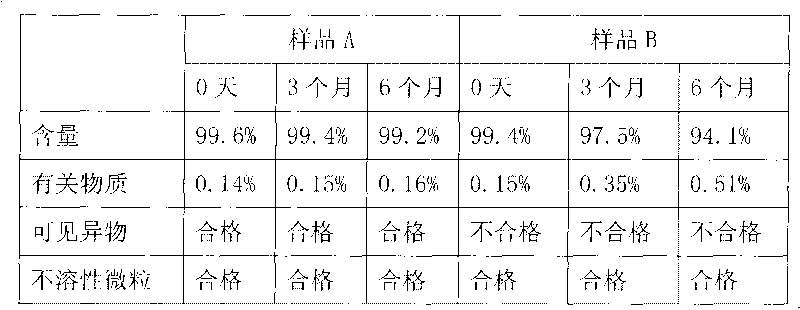
Image
Examples
Embodiment 1
[0037] Tirofiban hydrochloride (calculated as tirofiban) 1 serving
[0038] Polyethylene glycol 4000 0.5 part
[0039] Mannitol 10 servings
[0040] Sodium hydroxide solution (0.05mol / l) to adjust pH to 2.0
[0041] Preparation Process:
[0042] (1) Weigh tirofiban hydrochloride and polyethylene glycol 4000 of recipe quantity, dissolve in 30 ℃ of ethanol, 40 ℃ of rotary evaporations, prepare solid dispersion, add solid dispersion to water for injection, stir to dissolve, then Add mannitol, stir to dissolve, adjust the pH to 2.0 with sodium hydroxide solution, then filter and sterilize the medicinal liquid through a 0.22 μm microporous filter membrane, and fill the filtrate into a vial after passing the test, and then half stopper, Put in freeze-drying box.
[0043] (2) Turn on the freeze dryer to pre-freeze the product, reduce the temperature of the product to about -35°C at 2°C / min, maintain this temperature for 9 hours, and after the product is completely frozen, turn on...
Embodiment 2
[0046] Tirofiban hydrochloride (calculated as tirofiban) 1 serving
[0047] Povidone k30 5 servings
[0048] Dextran 40 0.5 parts
[0049] Potassium hydroxide solution (0.05mol / l) to adjust pH to 7.0
[0050] Preparation Process:
[0051] (1) Weigh tirofiban hydrochloride and povidone k30 of recipe quantity, dissolve in 75 ℃ of ethanol, 80 ℃ of rotary evaporations, prepare solid dispersion, add solid dispersion to water for injection, stir to dissolve, then add Dextran 40, stir to dissolve, adjust the pH to 7.0 with potassium hydroxide solution, then filter and sterilize the medicinal liquid through a 0.22 μm microporous membrane, and fill the filtrate into a vial after passing the test, then half stopper, put into the freeze-drying box.
[0052] (2) Turn on the lyophilizer to pre-freeze the product, reduce the temperature of the product to below about -60°C at 0.5°C / min, and keep this temperature for about 3 hours. After the product is completely frozen, start vacuuming for...
Embodiment 3
[0055] Tirofiban hydrochloride (calculated as tirofiban) 1 serving
[0056] 1 part succinic acid
[0058] Sodium hydroxide solution (0.05mol / l) to adjust pH to 8.0
[0059] Preparation Process:
[0060] (1) Weigh tirofiban hydrochloride and succinic acid of the recipe quantity, dissolve in 40°C 75% ethanol, rotate at 55°C to prepare a solid dispersion, add the solid dispersion to water for injection, stir to dissolve, then add Sodium chloride, stir to dissolve, adjust the pH to 8.0 with sodium hydroxide solution, then filter and sterilize the medicinal liquid through a 0.22 μm microporous membrane, and fill the filtrate into a vial after passing the test, and then half stopper, Put in freeze-drying box.
[0061] (2) Turn on the lyophilizer to pre-freeze the product, reduce the temperature of the product to about -50°C at 3°C / min, and maintain this temperature for about 4 hours. After the product is completely frozen, start vacuuming for free...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com