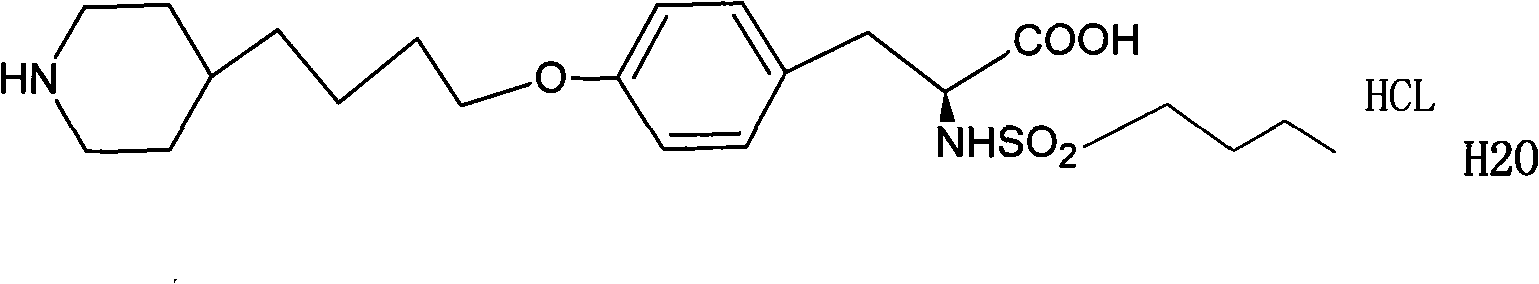
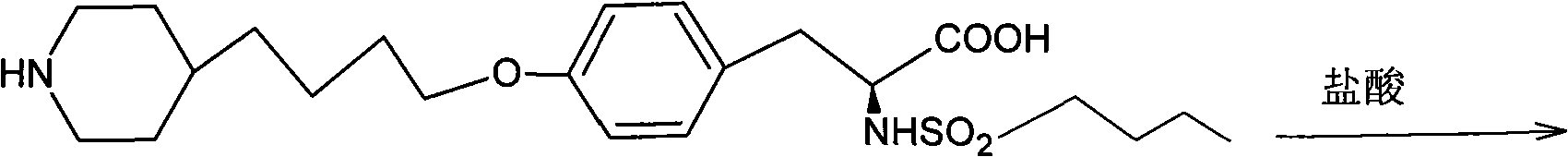
Preparation method of compound tirofiban hydrochloride
A technology of tirofiban and hydrochloric acid, applied in the field of pharmaceutical synthesis, can solve the problems of long process cycle, many solvents, isomerization in heating and concentration time, etc., and achieve the effect of reducing the side reaction of isomerization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Feeding amount:
[0037] N-n-Butylsulfonyl-O-4-(4’-pyridyl)butyl-L-tyrosine 65g
[0038] Palladium on carbon (10%) 5g
[0039] hydrogen
[0040] Ethanol solution (ethanol: water = 1:0.05) 1300ml
[0041] Concentrated hydrochloric acid 18ml
[0042] Process operation:
[0043] Put N-butylsulfonyl-O-4-(4'-pyridyl)butyl-L-tyrosine 65g, ethanol solution 1300ml, palladium carbon 5g into 2L hydrogenation kettle, add hydrochloric acid, and purge 5-10Kg of nitrogen / cm 2 , Use a pump to remove the nitrogen, repeat this twice, and then add hydrogen, slowly increase the temperature to 60-65℃, and the hydrogen pressure is 60Kg / cm 2, , Slowly start the stirring reaction for 8 hours, tap the plate, and measure the end point. When the end point is reached, discharge, filter, reduce the pressure of the filtrate to 1 / 8 of the original volume (measured water: alcohol: = 1:0.05), place the refrigerator, 5-10 ℃ crystallize, filter the next day, the filter cake is white , Washed twice with ethanol,...
Embodiment 2
[0056] Feeding amount:
[0057] N-n-Butylsulfonyl-O-4-(4’-pyridyl)butyl-L-tyrosine 65g
[0058] Palladium on carbon (10%) 5g
[0059] hydrogen
[0060] Methanol solution (methanol: water = 1:0.1) 1300ml
[0061] Concentrated hydrochloric acid 25ml
[0062] Process operation:
[0063] Put N-butylsulfonyl-O-4-(4'-pyridyl)butyl-L-tyrosine 65g, methanol solution 1300ml, palladium carbon 5g into 2L hydrogenation kettle, add hydrochloric acid, and purge 5-10Kg of nitrogen / cm 2 , Use a pump to remove the nitrogen, repeat this twice, and then add hydrogen, slowly increase the temperature to 60-65℃, and the hydrogen pressure is 60Kg / cm 2, , Slowly start the stirring reaction for 8 hours, tap the plate, and measure the end point. When the end point is reached, discharge, filter, reduce the pressure of the filtrate to 1 / 8 of the original volume (measured water: methanol: = 1:0.1), place in the refrigerator, crystallize at 5-10°C, filter the next day, the filter cake is white , Washed twice with ...
Embodiment 3
[0065] Feeding amount:
[0066] N-n-Butylsulfonyl-O-4-(4’-pyridyl)butyl-L-tyrosine 65g
[0067] Palladium on carbon (10%) 5g
[0068] hydrogen
[0069] Isopropanol solution (isopropanol: water = 1:0.1) 1300ml
[0070] Concentrated hydrochloric acid 20ml
[0071] Process operation:
[0072] Put N-butylsulfonyl-O-4-(4'-pyridyl)butyl-L-tyrosine 65g, isopropanol solution 1300ml, palladium carbon 5g into 2L hydrogenation kettle, add 20ml hydrochloric acid, and purge nitrogen 5-10Kg / cm 2 , Use a pump to remove the nitrogen, repeat this twice, and then add hydrogen, slowly increase the temperature to 65-70℃, and the hydrogen pressure 50Kg / cm 2, , Slowly start the stirring reaction for 8 hours, tap the plate, and measure the end point. When the end point is reached, discharge, filter, reduce the pressure of the filtrate to 1 / 5 of the original volume (measured water: isopropanol: = 1:0.2), place the refrigerator at 5-10 ℃ to crystallize, filter the next day, filter cake White, washed twice with...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com