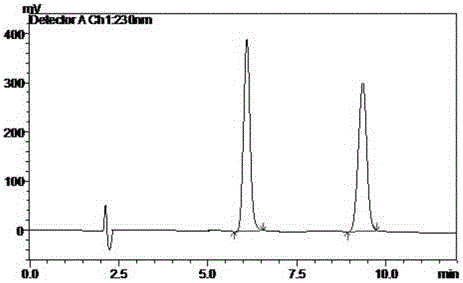
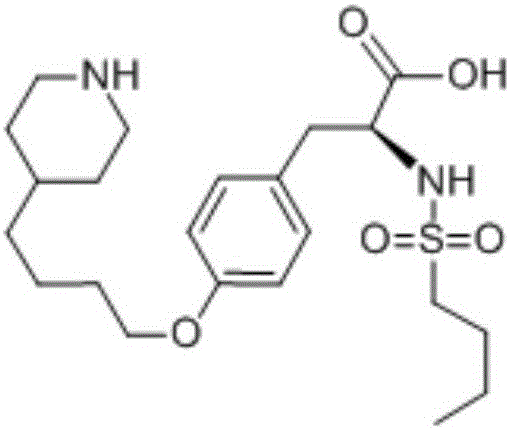
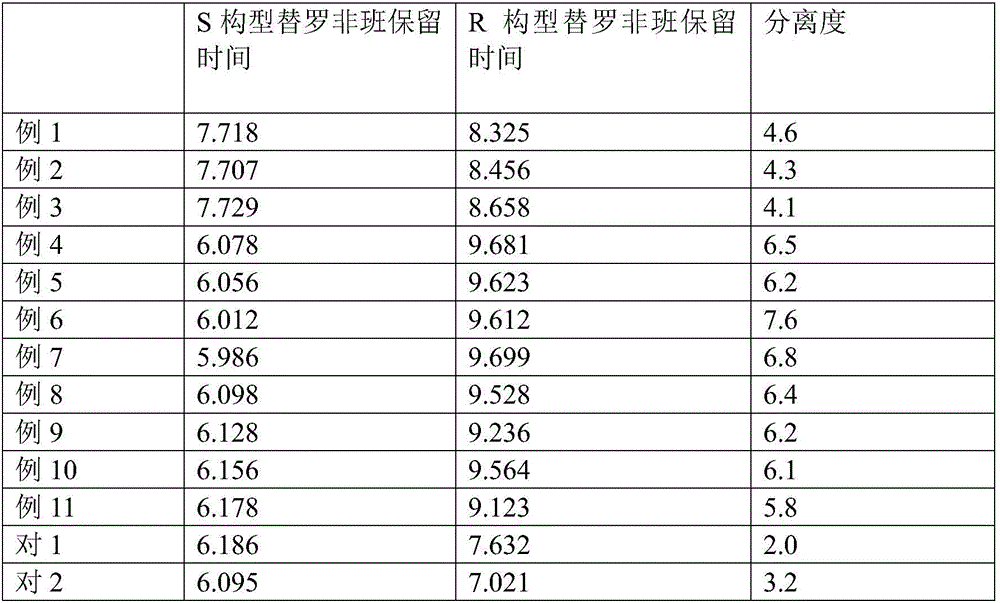
Method for separating and detecting cardio-cerebrovascular drug S-configuration tirofiban
A tirofiban and detection method technology, applied in the field of isomer separation, can solve the problems of increasing tirofiban separation cost, high cost of chiral chromatographic column, short service life, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0040] Preparation of Chitosan Modified Silica Gel Column Stationary Phase
[0041] Soak the silica gel in acid, and reflux, then wash with double distilled water until neutral, wash with acetone three times, bake to remove water and activate, cool and store in a desiccator for later use. Take the activated dry silica gel, add anhydrous dry toluene, and add KH-560 and triethylamine catalyst under stirring. in N 2Heating to reflux under protection, cooling, extracting with toluene, washing with acetone, methanol and acetone in sequence, and drying under vacuum to obtain a coupling agent-bonded silica gel material, and prepare a silica gel precursor.
[0042] Weigh the silica gel precursor, add chitosan, acetic acid and toluene to it under heating and stirring. Perchloric acid was added dropwise as catalyst, in N 2 Heating to reflux under protection, cooling, adjusting the pH to neutral, and precipitating solids to obtain the chitosan-modified silica gel chromatography colu...
Embodiment 1
[0061] Soak 10g of silica gel in 3mol / L hydrochloric acid, heat to reflux for 10h, then wash with twice distilled water until neutral, wash with acetone three times, bake at 150°C to remove water and activate for 8h, cool and store in a desiccator for later use. Take 6.0g of activated dry silica gel, add 100mL of anhydrous dry toluene, add 4.0mL of KH-560 and 3 drops of triethylamine catalyst under stirring. in N 2 Heating to reflux for 24 hours under protection, cooling, extracting with toluene for 24 hours, washing with acetone, methanol and acetone in sequence, and vacuum drying at 80°C for 8 hours to obtain a coupling agent-bonded silica gel material, and prepare a silica gel precursor.
[0062] Weigh 10 g of the silica gel precursor, add chitosan, acetic acid, and toluene therein under heating and stirring. 2 drops of perchloric acid were added dropwise as a catalyst, under N 2 Heating to reflux for 24 hours under protection, cooling, adjusting the pH to neutral, and pr...
Embodiment 2
[0065] Soak 10g of silica gel in 3mol / L hydrochloric acid, heat to reflux for 10h, then wash with twice distilled water until neutral, wash with acetone three times, bake at 150°C to remove water and activate for 8h, cool and store in a desiccator for later use. Take 6.0g of activated dry silica gel, add 100mL of anhydrous dry toluene, add 4.0mL of KH-560 and 3 drops of triethylamine catalyst under stirring. in N 2 Heating to reflux for 24 hours under protection, cooling, extracting with toluene for 24 hours, washing with acetone, methanol and acetone in sequence, and vacuum drying at 80°C for 8 hours to obtain a coupling agent-bonded silica gel material, and prepare a silica gel precursor.
[0066] Weigh 10 g of the silica gel precursor, add chitosan, acetic acid, and toluene therein under heating and stirring. 2 drops of perchloric acid were added dropwise as a catalyst, under N 2 Heating to reflux for 24 hours under protection, cooling, adjusting the pH to neutral, and pr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com