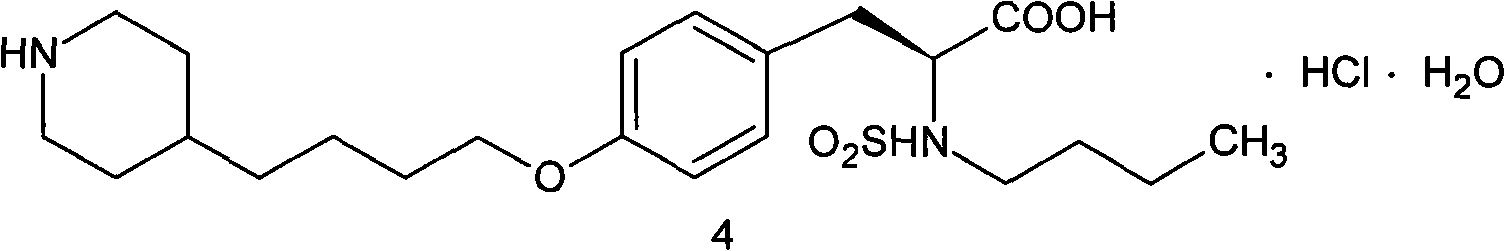
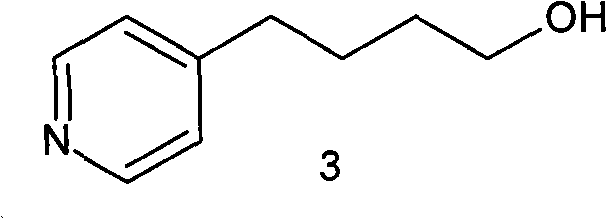
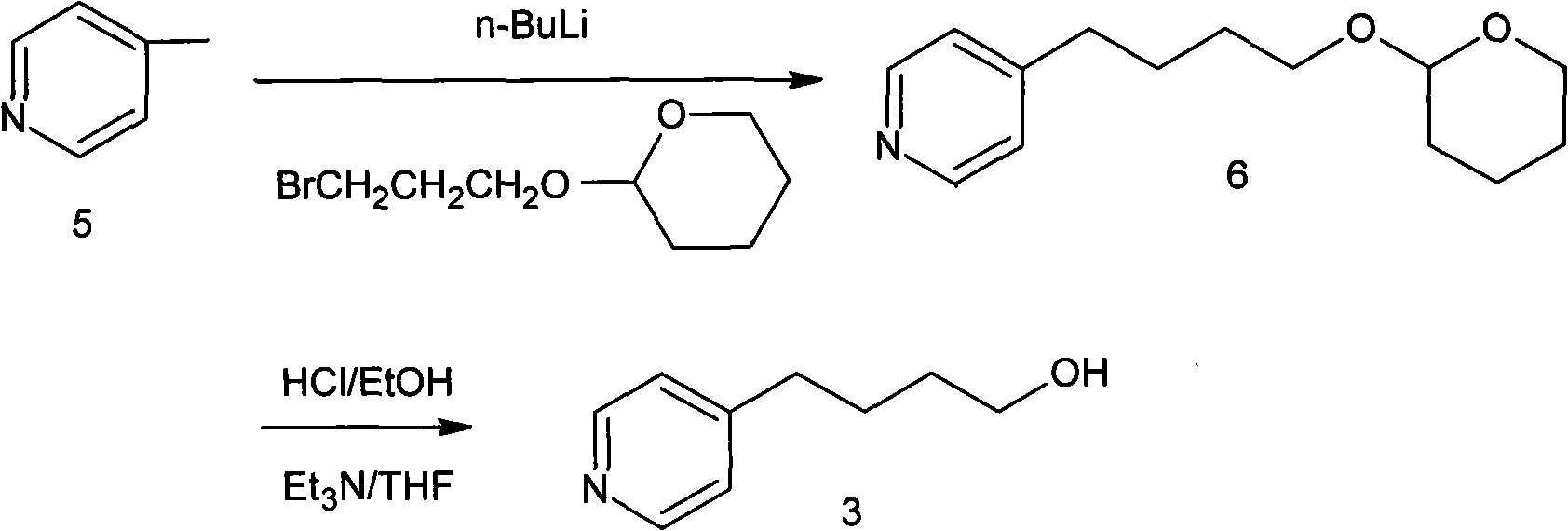
Method for preparing tirofiban hydrochloride intermediate
A technology for intermediates and inorganic salts, applied in the field of compound preparation, can solve the problems of difficulty in industrialized production of tirofiban hydrochloride, long production cycle, and difficulty in scale-up production, so as to avoid equipment corrosion, environmental pollution, and reaction steps. Short, method-efficient results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] The preparation of embodiment 14-(4-pyridyl)-1-butyric acid ethyl ester compound 2
[0037] Dissolve 500 grams of intermediate compound 1 in a mixed solvent of 1500 milliliters of dimethyl sulfoxide and 50 milliliters of water, add 120 grams of NaCl, raise the temperature to 140 ° C for 15 hours, and add 6 liters of water after detecting no raw material spots by thin-plate chromatography. After extraction with ethyl acetate, the organic layer was washed with saturated brine, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain compound 2 as a brownish-red oil, with a yield of 324.4 g and a yield of 89%.
[0038] 1 H NMR (400MHz, CD 3 SOCD 3 ): 1.22 (3H, t, J = 7.2Hz), 1.86 ~ 1.94 (2H, m), 2.31 (2H, t, J = 7.2Hz), 2.63 (2H, t, J = 7.6Hz), 4.05 ~ 4.10 (2H, m), 7.17 (2H, d, J = 4.8 Hz), 8.47 (2H, d, J = 4.0 Hz).
Embodiment 2
[0039] The preparation of embodiment 24-(4-pyridyl)-1-butanoic acid ethyl ester compound 2
[0040] Dissolve 500 grams of intermediate compound 1 in a mixed solvent of 2000 milliliters of dimethyl sulfoxide and 70 milliliters of water, add 120 grams of NaCl, heat up to 160 ° C for 10 hours, follow up and post-process as in Example 2 to obtain compound 2 , The amount was 336.2 grams, and the yield was 92%.
Embodiment 3
[0041] Example 3 Preparation of 4-(4-pyridyl)-1-butanoic acid ethyl ester compound 2
[0042] Dissolve 500 grams of intermediate compound 1 in a mixed solvent of 1500 milliliters of N,N-dimethylformamide and 50 milliliters of water, add 153 grams of KCl, heat up to 120° C. and react for 18 hours, follow up and follow up as in Example 2 Compound 2 was obtained after treatment, with a yield of 318.3 g and a yield of 87%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com