Medical freeze-dried sterile polyethylene film and preparation method thereof
A polyethylene film and polyethylene resin technology, which is applied in the field of pharmaceutical freeze-dried sterile polyethylene film and its preparation, can solve the problems of cross-contamination of drugs, difficult cleaning, and difficult detection of drug residues, and achieve strong mechanical properties, The effect of low insoluble particles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
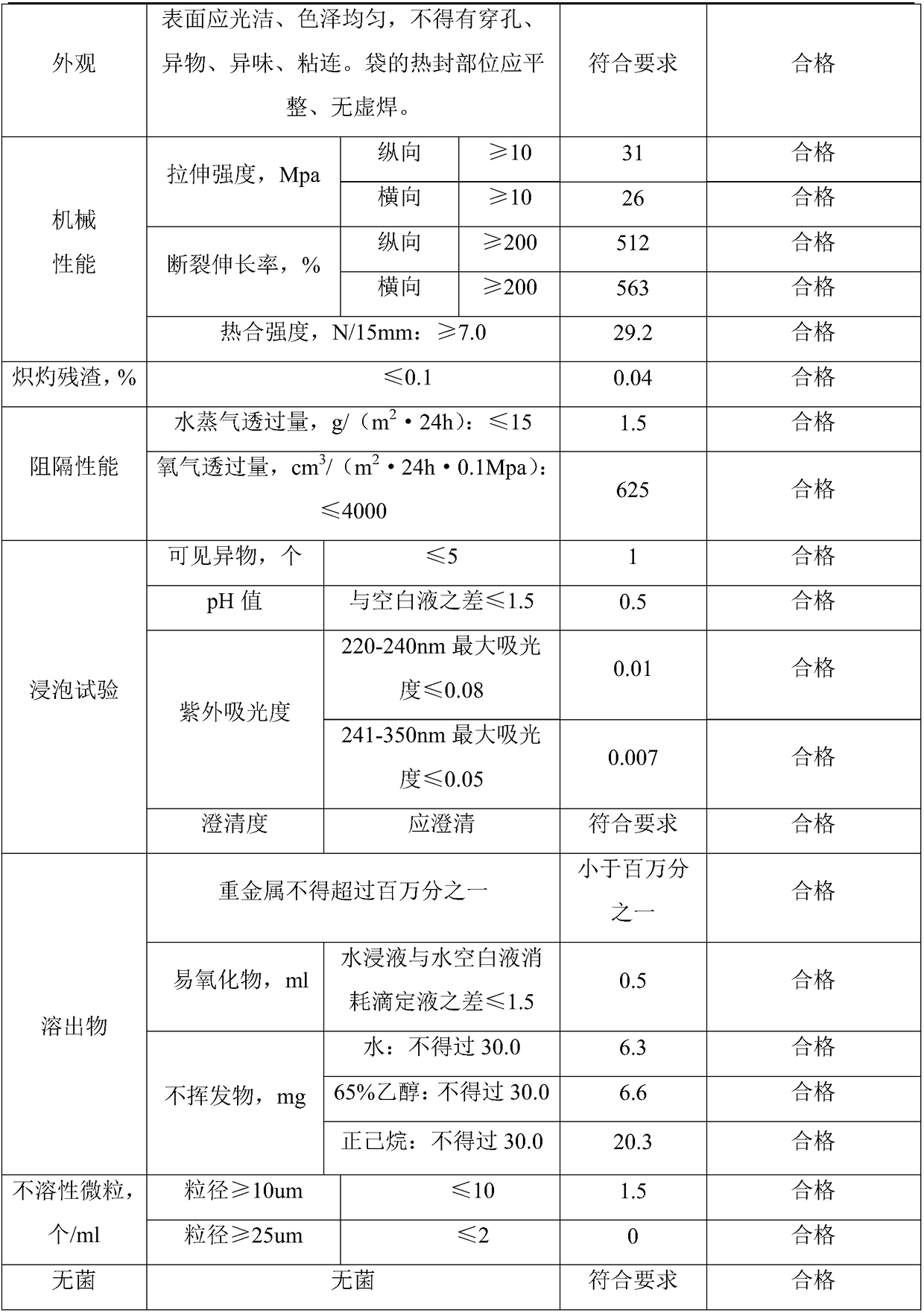
[0038] (1) Formula composition: 8 parts by weight of ultra-high molecular weight polyethylene resin with a melt index of 0.3 g / 10 min, and 2 parts by weight of metallocene polyethylene with a melt index of 0.3 g / 10 min.
[0039] (2) Mixing: Add the polyethylene resin of the above components into a mixer or blender, and mix for 15 minutes.
[0040] (3) Film making: Melt and blend uniformly mixed polyethylene resin through a film blowing machine. The temperature of the film blowing machine is controlled at 230°C, and extrusion blow molding is performed.
[0041] (4) Cutting film: Cut the roll film formed by extrusion blow molding according to the specification and size required by the customer.
[0042] (5) Packaging: The cut and formed polyethylene film is packed in more than two layers of pharmaceutical low-density polyethylene bags, and then packed in cartons or woven bags.
[0043] (6) Sterilization: use Co 60 γ-ray sterilization, the sterilization dose is 30KGy.
[0044]...
Embodiment 2
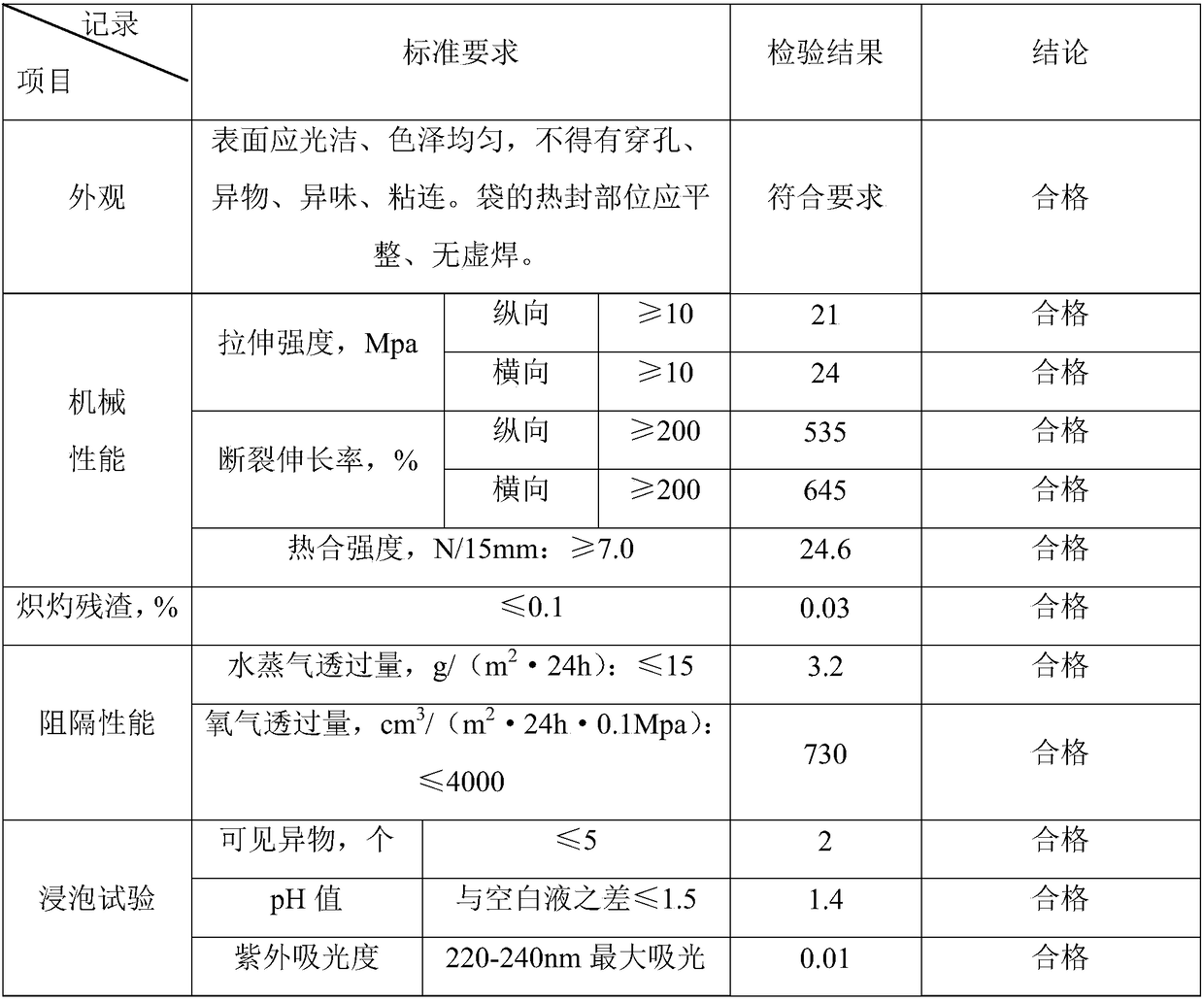
[0049] (1) Formula composition: 6 parts by weight of ultra-high molecular weight polyethylene resin with a melt index of 0.3 g / 10 min, and 4 parts by weight of metallocene polyethylene with a melt index of 0.3 g / 10 min.
[0050] (2) Mixing: Add the polyethylene resin of the above components into a mixer or blender, and mix for 15 minutes.
[0051] (3) Film making: Melt and blend uniformly mixed polyethylene resin through a film blowing machine. The temperature of the film blowing machine is controlled at 210° C., and extrusion blow molding is performed.
[0052] (4) Cutting film: Cut the roll film formed by extrusion blow molding according to the specification and size required by the customer.
[0053] (5) Packaging: The cut and formed polyethylene film is packed in more than two layers of pharmaceutical low-density polyethylene bags, and then packed in cartons or woven bags.
[0054] (6) Sterilization: use Co 60 Gamma-ray sterilization, the sterilization dose is 28KGy.
...
Embodiment 3
[0060] (1) Formula composition: 6 parts by weight of ultra-high molecular weight polyethylene resin with a melt index of 0.3g / 10min, 2 parts by weight of metallocene polyethylene with a melt index of 0.3g / 10min, and linear polyethylene resin with a melt index of 1.0g / 10min. 2 parts by weight of vinyl resin.
[0061] (2) Mixing: Add the polyethylene resin of the above components into a mixer or blender, and mix for 30 minutes.
[0062] (3) Film making: Melt and blend the homogeneously mixed polyethylene resin through a film blowing machine. The temperature of the film blowing machine is controlled at 190°C, and extrusion blow molding is performed.
[0063] (4) Cutting film: Cut the roll film formed by extrusion blow molding according to the specification and size required by the customer.
[0064] (5) Packaging: The cut and formed polyethylene film is packed in more than two layers of pharmaceutical low-density polyethylene bags, and then packed in cartons or woven bags.
[0...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melt flow index | aaaaa | aaaaa |
| melt flow index | aaaaa | aaaaa |
| melt flow index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com